Pharmacogenomic Profiling of Pediatric Acute Myeloid Leukemia to Identify Therapeutic Vulnerabilities and Inform Functional Precision Medicine
- PMID: 35960210
- PMCID: PMC9894568
- DOI: 10.1158/2643-3230.BCD-22-0011
Pharmacogenomic Profiling of Pediatric Acute Myeloid Leukemia to Identify Therapeutic Vulnerabilities and Inform Functional Precision Medicine
Abstract
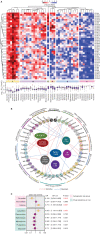
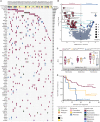
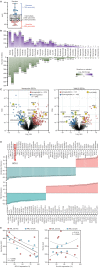
Despite the expanding portfolio of targeted therapies for adults with acute myeloid leukemia (AML), direct implementation in children is challenging due to inherent differences in underlying genetics. Here we established the pharmacologic profile of pediatric AML by screening myeloblast sensitivity to approved and investigational agents, revealing candidates of immediate clinical relevance. Drug responses ex vivo correlated with patient characteristics, exhibited age-specific alterations, and concorded with activities in xenograft models. Integration with genomic data uncovered new gene-drug associations, suggesting actionable therapeutic vulnerabilities. Transcriptome profiling further identified gene-expression signatures associated with on- and off-target drug responses. We also demonstrated the feasibility of drug screening-guided treatment for children with high-risk AML, with two evaluable cases achieving remission. Collectively, this study offers a high-dimensional gene-drug clinical data set that could be leveraged to research the unique biology of pediatric AML and sets the stage for realizing functional precision medicine for the clinical management of the disease.
Significance: We conducted integrated drug and genomic profiling of patient biopsies to build the functional genomic landscape of pediatric AML. Age-specific differences in drug response and new gene-drug interactions were identified. The feasibility of functional precision medicine-guided management of children with high-risk AML was successfully demonstrated in two evaluable clinical cases. This article is highlighted in the In This Issue feature, p. 476.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures



References
-
- Miranda-Filho A, Piñeros M, Ferlay J, Soerjomataram I, Monnereau A, Bray F. Epidemiological patterns of leukaemia in 184 countries: a population-based study. Lancet Haematol 2018;5:e14–24. - PubMed
-
- Rubnitz JE, Kaspers GJ. How I treat pediatric acute myeloid leukemia. Blood 2021;138:1009–18. - PubMed
-
- Kaspers GJ, Zimmermann M, Reinhardt D, Gibson BE, Tamminga RY, Aleinikova O, et al. . Improved outcome in pediatric relapsed acute myeloid leukemia: results of a randomized trial on liposomal daunorubicin by the International BFM Study Group. J Clin Oncol 2013;31:599–607. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

