Magnaporthe oryzae Chloroplast Targeting Endo-β-1,4-Xylanase I MoXYL1A Regulates Conidiation, Appressorium Maturation and Virulence of the Rice Blast Fungus
- PMID: 35960402
- PMCID: PMC9374862
- DOI: 10.1186/s12284-022-00584-2
Magnaporthe oryzae Chloroplast Targeting Endo-β-1,4-Xylanase I MoXYL1A Regulates Conidiation, Appressorium Maturation and Virulence of the Rice Blast Fungus
Abstract
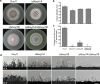
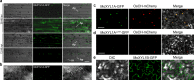
Endo-β-1,4-Xylanases are a group of extracellular enzymes that catalyze the hydrolysis of xylan, a principal constituent of the plant primary cell wall. The contribution of Endo-β-1,4-Xylanase I to both physiology and pathogenesis of the rice blast fungus M. oryzae is unknown. Here, we characterized the biological function of two endoxylanase I (MoXYL1A and MoXYL1B) genes in the development of M. oryzae using targeted gene deletion, biochemical analysis, and fluorescence microscopy. Phenotypic analysis of ∆Moxyl1A strains showed that MoXYL1A is required for the full virulence of M. oryzae but is dispensable for the vegetative growth of the rice blast fungus. MoXYL1B, in contrast, did not have a clear role in the infectious cycle but has a critical function in asexual reproduction of the fungus. The double deletion mutant was severely impaired in pathogenicity and virulence as well as asexual development. We found that MoXYL1A deletion compromised appressorium morphogenesis and function, leading to failure to penetrate host cells. Fluorescently tagged MoXYL1A and MoXYL1B displayed cytoplasmic localization in M. oryzae, while analysis of MoXYL1A-GFP and MoXYL1B-GFP in-planta revealed translocation and accumulation of these effector proteins into host cells. Meanwhile, sequence feature analysis showed that MoXYL1A possesses a transient chloroplast targeting signal peptide, and results from an Agrobacterium infiltration assay confirmed co-localization of MoXYL1A-GFP with ChCPN10C-RFP in the chloroplasts of host cells. MoXYL1B, accumulated to the cytoplasm of the host. Taken together, we conclude that MoXYL1A is a secreted effector protein that likely promotes the virulence of M. oryzae by interfering in the proper functioning of the host chloroplast, while the related xylanase MoXYL1B does not have a major role in virulence of M. oryzae.
Keywords: Chloroplast targeting peptide; Magnaporthe oryzae; Pathogenesis; Rice blast disease; Xylanases.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
MoGT2 Is Essential for Morphogenesis and Pathogenicity of Magnaporthe oryzae.mSphere. 2019 Sep 4;4(5):e00309-19. doi: 10.1128/mSphere.00309-19. mSphere. 2019. PMID: 31484736 Free PMC article.
-
Characterization of 47 Cys2 -His2 zinc finger proteins required for the development and pathogenicity of the rice blast fungus Magnaporthe oryzae.New Phytol. 2016 Aug;211(3):1035-51. doi: 10.1111/nph.13948. Epub 2016 Apr 4. New Phytol. 2016. PMID: 27041000
-
Large-scale gene disruption in Magnaporthe oryzae identifies MC69, a secreted protein required for infection by monocot and dicot fungal pathogens.PLoS Pathog. 2012;8(5):e1002711. doi: 10.1371/journal.ppat.1002711. Epub 2012 May 10. PLoS Pathog. 2012. PMID: 22589729 Free PMC article.
-
Investigating the cell and developmental biology of plant infection by the rice blast fungus Magnaporthe oryzae.Fungal Genet Biol. 2021 Sep;154:103562. doi: 10.1016/j.fgb.2021.103562. Epub 2021 Apr 18. Fungal Genet Biol. 2021. PMID: 33882359 Review.
-
The role of glycerol in the pathogenic lifestyle of the rice blast fungus Magnaporthe oryzae.Environ Microbiol. 2017 Mar;19(3):1008-1016. doi: 10.1111/1462-2920.13688. Epub 2017 Mar 1. Environ Microbiol. 2017. PMID: 28165657 Review.
Cited by
-
Whole-Genome Sequencing and Genome Annotation of Pathogenic Elsinoë batatas Causing Stem and Foliage Scab Disease in Sweet Potato.J Fungi (Basel). 2024 Dec 18;10(12):882. doi: 10.3390/jof10120882. J Fungi (Basel). 2024. PMID: 39728378 Free PMC article.
-
Dual-RNA-sequencing to elucidate the interactions between sorghum and Colletotrichum sublineola.Front Fungal Biol. 2024 Aug 16;5:1437344. doi: 10.3389/ffunb.2024.1437344. eCollection 2024. Front Fungal Biol. 2024. PMID: 39220294 Free PMC article.
-
Global characterization of GH11 family xylanases genes in Neostagonosporella sichuanensis and functional analysis of Nsxyn1 and Nsxyn2.Front Microbiol. 2024 Nov 21;15:1507998. doi: 10.3389/fmicb.2024.1507998. eCollection 2024. Front Microbiol. 2024. PMID: 39640849 Free PMC article.
-
The phase-separating Magnaporthe oryzae MoSpa2 complex organizes actin nucleation centers for plant infection.Plant Cell. 2025 May 9;37(5):koaf097. doi: 10.1093/plcell/koaf097. Plant Cell. 2025. PMID: 40315356
-
Appressoria-Small but Incredibly Powerful Structures in Plant-Pathogen Interactions.Int J Mol Sci. 2023 Jan 21;24(3):2141. doi: 10.3390/ijms24032141. Int J Mol Sci. 2023. PMID: 36768468 Free PMC article. Review.
References
-
- Akagi A, Jiang C-J, Takatsuji H. Magnaporthe oryzae inoculation of rice seedlings by spraying with a spore suspension. Bio-Protoc. 2015;5(11):e1486–e1486. doi: 10.21769/BioProtoc.1486. - DOI
-
- Aliyu SR, Lin L, Chen X, Abdul W, Lin Y, Otieno FJ, Shabbir A, Batool W, Zhang Y, Tang W, Tang W. Disruption of putative short-chain acyl-CoA dehydrogenases compromised free radical scavenging, conidiogenesis, and pathogenesis of Magnaporthe oryzae. Fungal Genet Biol. 2019;127:23–34. doi: 10.1016/j.fgb.2019.02.010. - DOI - PubMed
-
- Annis SL, Goodwin PH. Recent advances in the molecular genetics of plant cell wall-degrading enzymes produced by plant pathogenic fungi. Eur J Plant Pathol. 1997;103(1):1–14. doi: 10.1023/A:1008656013255. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

