Comparative Study of Elabela and Apelin on Apelin Receptor Activation Through β-Arrestin Recruitment
- PMID: 35960440
- PMCID: PMC9935735
- DOI: 10.1007/s12033-022-00529-6
Comparative Study of Elabela and Apelin on Apelin Receptor Activation Through β-Arrestin Recruitment
Abstract
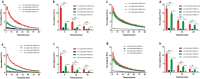
Apelin receptor (APJ) ligands elabela (ELA) and apelin have divergent distributions and function differently in vitro and in vivo. Whether differences exist in their capacity of recruitment of β-arrestins (ARRBs) to APJ remains unknown. The aim of the current study was to investigate the different effects of ELA and apelin on the interaction between APJ and ARRBs in live cells by NanoBiT®. NanoBiT® system is a new technology for studying protein-protein interaction in real-time in live cells, based on the emission of luminescence when two split components of NanoLuc luciferase, large Bit (LgBit) and small Bit (SmBit), complement each other to form an enzymatically active entity. We tagged the APJ and ARRBs with LgBit or SmBit and then evaluated their interactions in transiently transfected HEK293T cells, and determined the signal strength yielded as a result of the interaction. We also investigated the concentration-dependent response of the APJ-ARRB interaction in response to ELA and apelin. Finally, we assessed the effect of F13A, an APJ antagonist which is structurally very similar to apelin-13, on ELA- and apelin-mediated APJ-ARRB interactions. The NanoLuc® luciferase signal was highest in the pair of APJ-LgBit with SmBit-ARRB1 or SmBit-ARRB2. NanoLuc® luciferase signal increased in a concentration-dependent manner from 0.1 nM to 10 μM in response to ELA or apelin. Interestingly, ELA elicited weaker APJ-ARRB interaction signals than apelin. Pre-treatment with F13A potently reduced the APJ-ARRB interaction in response to both ELA and apelin. Our results demonstrated that both ELA and apelin promoted the interaction of APJ and ARRBs in a concentration-dependent manner, and ELA is less efficacious than apelin in inducing the recruitment of ARRBs to APJ, providing a biased functional aspect of ELA vs. apelin at the receptor signaling level. Additionally, ELA and apelin may share the same binding site(s) or pocket(s) at the APJ level.
Keywords: ARRBs; Apelin; Elebela/Toddler; NanoBiT®; The apelin receptor (APJ).
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
ELABELA-APJ axis protects from pressure overload heart failure and angiotensin II-induced cardiac damage.Cardiovasc Res. 2017 Jun 1;113(7):760-769. doi: 10.1093/cvr/cvx061. Cardiovasc Res. 2017. PMID: 28371822
-
Individual phosphorylation sites at the C-terminus of the apelin receptor play different roles in signal transduction.Redox Biol. 2020 Sep;36:101629. doi: 10.1016/j.redox.2020.101629. Epub 2020 Jun 30. Redox Biol. 2020. PMID: 32863206 Free PMC article.
-
Apelin and Elabela/Toddler; double ligands for APJ/Apelin receptor in heart development, physiology, and pathology.Peptides. 2019 Jan;111:62-70. doi: 10.1016/j.peptides.2018.04.011. Epub 2018 Apr 21. Peptides. 2019. PMID: 29684595 Review.
-
Therapeutic potential of apelin and Elabela in cardiovascular disease.Biomed Pharmacother. 2023 Oct;166:115268. doi: 10.1016/j.biopha.2023.115268. Epub 2023 Aug 8. Biomed Pharmacother. 2023. PMID: 37562237 Review.
-
Structure-Activity Relationship and Bioactivity of Short Analogues of ELABELA as Agonists of the Apelin Receptor.J Med Chem. 2021 Jan 14;64(1):602-615. doi: 10.1021/acs.jmedchem.0c01547. Epub 2020 Dec 22. J Med Chem. 2021. PMID: 33350824
Cited by
-
Evidence for the Induction of Analgesic Cross-Tolerance Between Opioid and Apelin/APJ Systems in Male Rats.J Stud Alcohol Drugs. 2024 Sep;85(5):704-712. doi: 10.15288/jsad.23-00377. Epub 2024 Mar 22. J Stud Alcohol Drugs. 2024. PMID: 38517751 Free PMC article.
-
Integration of RRBS and RNA-seq unravels the regulatory role of DNMT3A in porcine Sertoli cell proliferation.Front Genet. 2024 Jan 9;14:1302351. doi: 10.3389/fgene.2023.1302351. eCollection 2023. Front Genet. 2024. PMID: 38264208 Free PMC article.
-
Apelinergic System Affects Electrocardiographic Abnormalities Induced by Doxorubicin.Biomedicines. 2025 Jan 3;13(1):94. doi: 10.3390/biomedicines13010094. Biomedicines. 2025. PMID: 39857678 Free PMC article.
References
-
- Read C, Nyimanu D, Williams TL, Huggins DJ, Sulentic P, Macrae RGC, Yang P, Glen RC, Maguire JJ, Davenport AP. International union of basic and clinical pharmacology. CVII. Structure and pharmacology of the apelin receptor with a recommendation that elabela/toddler is a second endogenous peptide ligand. Pharmacological Reviews. 2019;71(4):467–502. doi: 10.1124/pr.119.017533. - DOI - PMC - PubMed
-
- Taheri S, Murphy K, Cohen M, Sujkovic E, Kennedy A, Dhillo W, Dakin C, Sajedi A, Ghatei M, Bloom S. The effects of centrally administered apelin-13 on food intake, water intake and pituitary hormone release in rats. Biochemical and Biophysical Research Communications. 2002;291(5):1208–1212. doi: 10.1006/bbrc.2002.6575. - DOI - PubMed
-
- Owen NE, Nyimanu D, Kuc RE, Upton PD, Morrell NW, Alexander GJ, Maguire JJ, Davenport AP. Plasma levels of apelin are reduced in patients with liver fibrosis and cirrhosis but are not correlated with circulating levels of bone morphogenetic protein 9 and 10. Peptides. 2021;136:170440. doi: 10.1016/j.peptides.2020.170440. - DOI - PMC - PubMed
-
- Yamazaki S, Sekiguchi A, Uchiyama A, Fujiwara C, Inoue Y, Yokoyama Y, Ogino S, Torii R, Hosoi M, Akai R, et al. Apelin/APJ signaling suppresses the pressure ulcer formation in cutaneous ischemia-reperfusion injury mouse model. Scientific Reports. 2020;10(1):1349. doi: 10.1038/s41598-020-58452-2. - DOI - PMC - PubMed
-
- Hus-Citharel A, Bodineau L, Frugiere A, Joubert F, Bouby N, Llorens-Cortes C. Apelin counteracts vasopressin-induced water reabsorption via cross talk between apelin and vasopressin receptor signaling pathways in the rat collecting duct. Endocrinology. 2014;155(11):4483–4493. doi: 10.1210/en.2014-1257. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

