Tolerability of COVID-19 Infection and Messenger RNA Vaccination Among Patients With a History of Kawasaki Disease
- PMID: 35960521
- PMCID: PMC9375169
- DOI: 10.1001/jamanetworkopen.2022.26236
Tolerability of COVID-19 Infection and Messenger RNA Vaccination Among Patients With a History of Kawasaki Disease
Abstract
Importance: Kawasaki disease (KD) symptoms significantly overlap with multisystem inflammatory syndrome in children due to COVID-19. Patients with KD may be at risk for adverse outcomes from exposure to SARS-CoV-2 infection or vaccination.
Objective: To describe the outcomes of patients with KD to SARS-CoV-2 infection or vaccination.
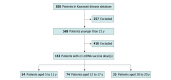
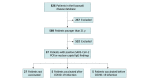
Design, setting, and participants: This case series evaluated 2 cohorts using an existing KD database and reviewed individual electronic medical records for the period spanning January 1, 2020, through January 31, 2022, via electronic medical records that include Washington state immunization records. Vaccine cohort inclusion criteria consisted of being 21 years or younger at immunization and receiving 1 or more BNT162b2 (Pfizer-BioNTech) or messenger RNA (mRNA)-1273 (Moderna) vaccine doses. The COVID-19 cohort included patients 21 years or younger with positive polymerase chain reaction or nuclear capsid IgG findings for SARS-CoV-2. Participants included 826 patients from a preexisting KD database. One hundred fifty-three patients received at least 1 BNT162b2 or mRNA-1273 vaccine dose and were included in the mRNA vaccine cohort. Thirty-seven patients had positive test results for SARS-CoV-2 and were included in the COVID-19 cohort.
Exposures: SARS-CoV-2 vaccination and/or infection.
Main outcomes and measures: Adverse events after mRNA vaccination and/or COVID-19, including clinician visits, emergency department encounters, or hospitalizations.
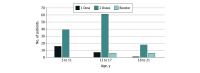
Results: Among the 153 patients included in the mRNA vaccination cohort (mean [SD] age, 13.0 [4.3] years; 94 male [61.4%]), the BNT162b2 vaccine was provided for 143 (93.5%), and the remaining 10 (6.5%) received mRNA-1273 or a combination of both. Among patients in the vaccine cohort, 129 (84.3%) were fully vaccinated or received a third-dose booster. No clinically severe adverse events occurred, and there were no reports of vaccine-related hospitalizations or outpatient visits. The COVID-19 cohort included 37 patients (mean [SD] age, 11.0 [5.5] years; 22 male [59.5%]). No patients required hospitalization due to COVID-19. The most common symptoms included low-grade fever, fatigue, cough, and myalgia with resolution within a few days. Two patients, aged 9 and 19 years, had extended cough and fatigue for 3 to 4 weeks. One patient developed COVID-19 within 6 weeks of receiving intravenous immunoglobulin for KD.
Conclusions and relevance: These findings suggest that the mRNA vaccines may be safe and COVID-19 may not be severe for patients with a history of KD.
Conflict of interest statement
Figures
References
-
- McCrindle BW, Rowley AH, Newburger JW, et al. ; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention . Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927-e999. doi:10.1161/CIR.0000000000000484 - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

