Triple-negative breast cancer influences a mixed M1/M2 macrophage phenotype associated with tumor aggressiveness
- PMID: 35960749
- PMCID: PMC9374254
- DOI: 10.1371/journal.pone.0273044
Triple-negative breast cancer influences a mixed M1/M2 macrophage phenotype associated with tumor aggressiveness
Abstract
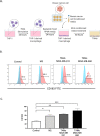
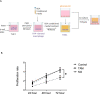
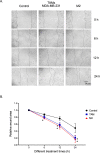
Triple-negative breast cancer (TNBC) is characterized by excessive accumulation of tumor-infiltrating immune cells, including tumor-associated macrophages (TAMs). TAMs consist of a heterogeneous population with high plasticity and are associated with tumor aggressiveness and poor prognosis. Moreover, breast cancer cells can secrete factors that influence TAM polarization. Therefore, this study aimed to evaluate the crosstalk between cancer cells and macrophages in the context of TNBC. Cytokine-polarized M2 macrophage were used as control. Distinct from the classical M2 macrophage, TAMs generated from TNBC-conditioned media upregulated both M1- and M2-associated genes, and secreted both the anti-inflammatory cytokine interleukin IL-10 and the proinflammatory cytokine IL-6 and tumor necrosis factor- α. Theses TNBC-induced TAMs exert aggressive behavior of TNBC cells. Consistently, TCGA and MTABRIC analyses of human breast cancer revealed upregulation of M1- associated genes in TNBC comparing with non-TNBC. Among these M1-associated genes, CXCL10 and IL1B were revealed to be independent prognostic factors for disease progression. In conclusion, TNBC cells induce macrophage polarization with a mixture of M1 and M2 phenotypes. These cancer-induced TAMs further enhance tumor cell growth and aggressiveness.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Taraxacum mongolicum extract inhibited malignant phenotype of triple-negative breast cancer cells in tumor-associated macrophages microenvironment through suppressing IL-10 / STAT3 / PD-L1 signaling pathways.J Ethnopharmacol. 2021 Jun 28;274:113978. doi: 10.1016/j.jep.2021.113978. Epub 2021 Mar 11. J Ethnopharmacol. 2021. PMID: 33716082
-
The Effect of Salvianolic Acid A on Tumor-Associated Macrophage Polarization and Its Mechanisms in the Tumor Microenvironment of Triple-Negative Breast Cancer.Molecules. 2024 Mar 26;29(7):1469. doi: 10.3390/molecules29071469. Molecules. 2024. PMID: 38611749 Free PMC article.
-
Tumor-associated macrophages of the M1/M2 phenotype are involved in the regulation of malignant biological behavior of breast cancer cells through the EMT pathway.Med Oncol. 2022 May 16;39(5):83. doi: 10.1007/s12032-022-01670-7. Med Oncol. 2022. PMID: 35570226
-
Triple negative breast cancer: Key role of Tumor-Associated Macrophages in regulating the activity of anti-PD-1/PD-L1 agents.Biochim Biophys Acta Rev Cancer. 2018 Jan;1869(1):78-84. doi: 10.1016/j.bbcan.2017.10.007. Epub 2017 Nov 7. Biochim Biophys Acta Rev Cancer. 2018. PMID: 29126881 Review.
-
The role of tumor-associated macrophage in breast cancer biology.Histol Histopathol. 2018 Feb;33(2):133-145. doi: 10.14670/HH-11-916. Epub 2017 Jul 6. Histol Histopathol. 2018. PMID: 28681373 Review.
Cited by
-
Glutamine-derived aspartate is required for eIF5A hypusination-mediated translation of HIF-1α to induce the polarization of tumor-associated macrophages.Exp Mol Med. 2024 May;56(5):1123-1136. doi: 10.1038/s12276-024-01214-1. Epub 2024 May 1. Exp Mol Med. 2024. PMID: 38689086 Free PMC article.
-
Development of 42 marker panel for in-depth study of cancer associated fibroblast niches in breast cancer using imaging mass cytometry.Front Immunol. 2024 Apr 22;15:1325191. doi: 10.3389/fimmu.2024.1325191. eCollection 2024. Front Immunol. 2024. PMID: 38711512 Free PMC article.
-
Tumor-associated macrophages correlate with better outcome in SHH medulloblastoma.Front Oncol. 2025 Apr 15;15:1557313. doi: 10.3389/fonc.2025.1557313. eCollection 2025. Front Oncol. 2025. PMID: 40303994 Free PMC article.
-
The Role of Tumor Epithelial-Mesenchymal Transition and Macrophage Crosstalk in Cancer Progression.Curr Osteoporos Rep. 2023 Apr;21(2):117-127. doi: 10.1007/s11914-023-00780-z. Epub 2023 Feb 27. Curr Osteoporos Rep. 2023. PMID: 36848026 Free PMC article. Review.
-
The Interplay between Atherosclerosis and Cancer: Breast Cancer Cells Increase the Expression of Endothelial Cell Adhesion Markers.Biology (Basel). 2023 Jun 22;12(7):896. doi: 10.3390/biology12070896. Biology (Basel). 2023. PMID: 37508329 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

