Bioinspired large Stokes shift small molecular dyes for biomedical fluorescence imaging
- PMID: 35960804
- PMCID: PMC9374339
- DOI: 10.1126/sciadv.abo3289
Bioinspired large Stokes shift small molecular dyes for biomedical fluorescence imaging
Abstract
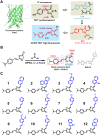
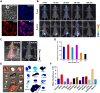
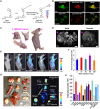
Long Stokes shift dyes that minimize cross-talk between the excitation source and fluorescent emission to improve the signal-to-background ratio are highly desired for fluorescence imaging. However, simple small molecular dyes with large Stokes shift (more than 120 nanometers) and near-infrared (NIR) emissions have been rarely reported so far. Here, inspired by the chromophore chemical structure of fluorescent proteins, we designed and synthesized a series of styrene oxazolone dyes (SODs) with simple synthetic methods, which show NIR emissions (>650 nanometers) with long Stokes shift (ranged from 136 to 198 nanometers) and small molecular weight (<450 daltons). The most promising SOD9 shows rapid renal excretion and blood-brain barrier passing properties. After functioning with the mitochondrial-targeted triphenylphosphonium (TPP) group, the resulting SOD9-TPP can be engineered for head-neck tumor imaging, fluorescence image-guided surgery, brain neuroimaging, and on-site pathologic analysis. In summary, our findings add an essential small molecular dye category to the classical dyes.
Figures



References
-
- Huang J., Pu K., Activatable molecular probes for second near-infrared fluorescence, chemiluminescence, and photoacoustic imaging. Angew. Chem. Int. Ed. Engl. 59, 11717–11731 (2020). - PubMed
-
- Hong G., Antaris A. L., Dai H., Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 1, 0010 (2017).
-
- Huang J., Xie C., Zhang X., Jiang Y., Li J., Fan Q., Pu K., Renal-clearable molecular semiconductor for second near-infrared fluorescence imaging of kidney dysfunction. Angew. Chem. Int. Ed. Engl. 58, 15120–15127 (2019). - PubMed
-
- Zhang J., Campbell R. E., Ting A. Y., Tsien R. Y., Creating new fluorescent probes for cell biology. Nat. Rev. Mol. Cell Biol. 3, 906–918 (2002). - PubMed
-
- Cheng P., Pu K., Molecular imaging and disease theranostics with renal-clearable optical agents. Nat. Rev. Mater. 6, 1095–1113 (2021).
LinkOut - more resources
Full Text Sources
Miscellaneous

