A model of painful vaso-occlusive crisis in mice with sickle cell disease
- PMID: 35960856
- PMCID: PMC9837430
- DOI: 10.1182/blood.2022017309
A model of painful vaso-occlusive crisis in mice with sickle cell disease
Conflict of interest statement
Figures
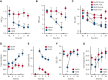
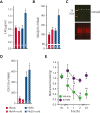
References
-
- Darbari DS, Sheehan VA, Ballas SK. The vaso-occlusive pain crisis in sickle cell disease: definition, pathophysiology, and management. Eur J Haematol. 2020;105(3):237–246. - PubMed
-
- Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood. 2012;120(18):3647–3656. - PubMed
-
- Pászty C, Brion CM, Manci E, et al. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278(5339):876–878. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

