Cascade Testing for Hereditary Cancer Syndromes: Should We Move Toward Direct Relative Contact? A Systematic Review and Meta-Analysis
- PMID: 35960887
- PMCID: PMC9746789
- DOI: 10.1200/JCO.22.00303
Cascade Testing for Hereditary Cancer Syndromes: Should We Move Toward Direct Relative Contact? A Systematic Review and Meta-Analysis
Abstract
Purpose: Evidence-based guidelines recommend cascade genetic counseling and testing for hereditary cancer syndromes, providing relatives the opportunity for early detection and prevention of cancer. The current standard is for patients to contact and encourage relatives (patient-mediated contact) to undergo counseling and testing. Direct relative contact by the medical team or testing laboratory has shown promise but is complicated by privacy laws and lack of infrastructure. We sought to compare outcomes associated with patient-mediated and direct relative contact for hereditary cancer cascade genetic counseling and testing in the first meta-analysis on this topic.
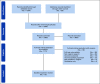
Materials and methods: We conducted a systematic review and meta-analysis in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PROSPERO No.: CRD42020134276). We searched key electronic databases to identify studies evaluating hereditary cancer cascade testing. Eligible trials were subjected to meta-analysis.
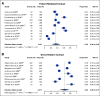
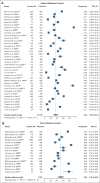
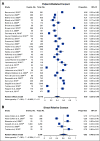
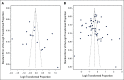
Results: Eighty-seven studies met inclusion criteria. Among relatives included in the meta-analysis, 48% (95% CI, 38 to 58) underwent cascade genetic counseling and 41% (95% CI, 34 to 48) cascade genetic testing. Compared with the patient-mediated approach, direct relative contact resulted in significantly higher uptake of genetic counseling for all relatives (63% [95% CI, 49 to 75] v 35% [95% CI, 24 to 48]) and genetic testing for first-degree relatives (62% [95% CI, 49 to 73] v 40% [95% CI, 32 to 48]). Methods of direct contact included telephone calls, letters, and e-mails; respective rates of genetic testing completion were 61% (95% CI, 51 to 70), 48% (95% CI, 37 to 59), and 48% (95% CI, 45 to 50).
Conclusion: Most relatives at risk for hereditary cancer do not undergo cascade genetic counseling and testing, forgoing potentially life-saving medical interventions. Compared with patient-mediated contact, direct relative contact increased rates of cascade genetic counseling and testing, arguing for a shift in the care delivery paradigm, to be confirmed by randomized controlled trials.
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures
References
-
- Tuffaha HW, Mitchell A, Ward RL, et al. : Cost-effectiveness analysis of germ-line BRCA testing in women with breast cancer and cascade testing in family members of mutation carriers. Genet Med 20:985-994, 2018 - PubMed
-
- Committee on Gynecologic Practice : ACOG Committee Opinion No. 727: Cascade testing: Testing women for known hereditary genetic mutations associated with cancer. Obstet Gynecol 131:e31-e34, 2018 - PubMed
-
- Randall LM, Pothuri B, Swisher EM, et al. : Multi-disciplinary summit on genetics services for women with gynecologic cancers: A Society of Gynecologic Oncology White Paper. Gynecol Oncol 146:217-224, 2017 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

