Unraveling the Pivotal Role of Atropisomerism for Cellular Internalization
- PMID: 35960892
- PMCID: PMC9446767
- DOI: 10.1021/jacs.2c05844
Unraveling the Pivotal Role of Atropisomerism for Cellular Internalization
Abstract
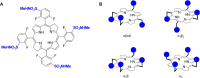
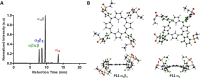
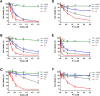
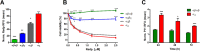
The intrinsic challenge of large molecules to cross the cell membrane and reach intracellular targets is a major obstacle for the development of new medicines. We report how rotation along a single C-C bond, between atropisomers of a drug in clinical trials, improves cell uptake and therapeutic efficacy. The atropisomers of redaporfin (a fluorinated sulfonamide bacteriochlorin photosensitizer of 1135 Da) are separable and display orders of magnitude differences in photodynamic efficacy that are directly related to their differential cellular uptake. We show that redaporfin atropisomer uptake is passive and only marginally affected by ATP depletion, plasma proteins, or formulation in micelles. The α4 atropisomer, where meso-phenyl sulfonamide substituents are on the same side of the tetrapyrrole macrocycle, exhibits the highest cellular uptake and phototoxicity. This is the most amphipathic atropisomer with a conformation that optimizes hydrogen bonding (H-bonding) with polar head groups of membrane phospholipids. Consequently, α4 binds to the phospholipids on the surface of the membrane, flips into the membrane to adopt the orientation of a surfactant, and eventually diffuses to the interior of the cell (bind-flip mechanism). We observed increased α4 internalization by cells of the tumor microenvironment in vivo and correlated this to the response of photodynamic therapy when tumor illumination was performed 24 h after α4 administration. These results show that properly orientated aryl sulfonamide groups can be incorporated into drug design as efficient cell-penetrating motifs in vivo and reveal the unexpected biological consequences of atropisomerism.
Conflict of interest statement
The authors declare the following competing financial interest(s): F.S., N.G., M.P., and L.G.A. have patents on redaporfin licensed to Luzitin SA. N.G. was an employee of Luzitin and is now an independent researcher at the University of Aveiro.
Figures

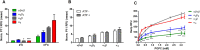
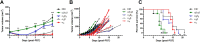

References
-
- Sakamoto K.; Michibata J.; Hirai Y.; Ide A.; Ikitoh A.; Takatani-Nakase T.; Futaki S. Potentiating the Membrane Interaction of an Attenuated Cationic Amphiphilic Lytic Peptide for Intracellular Protein Delivery by Anchoring with Pyrene Moiety. Bioconjugate Chem. 2021, 32, 950–957. 10.1021/acs.bioconjchem.1c00101. - DOI - PubMed
-
- Allen J.; Najjar K.; Erazo-Oliveras A.; Kondow-McConaghy H. M.; Brock D. J.; Graham K.; Hager E. C.; Marschall A. L. J.; Dübel S.; Juliano R. L.; Pellois J.-P. Cytosolic Delivery of Macromolecules in Live Human Cells Using the Combined Endosomal Escape Activities of a Small Molecule and Cell Penetrating Peptides. ACS Chem. Biol. 2019, 14, 2641–2651. 10.1021/acschembio.9b00585. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

