Elotuzumab Plus Pomalidomide and Dexamethasone for Relapsed/Refractory Multiple Myeloma: Final Overall Survival Analysis From the Randomized Phase II ELOQUENT-3 Trial
- PMID: 35960908
- PMCID: PMC9870233
- DOI: 10.1200/JCO.21.02815
Elotuzumab Plus Pomalidomide and Dexamethasone for Relapsed/Refractory Multiple Myeloma: Final Overall Survival Analysis From the Randomized Phase II ELOQUENT-3 Trial
Abstract
Purpose: In the phase II ELOQUENT-3 trial (ClinicalTrials.gov identifier: NCT02654132), elotuzumab combined with pomalidomide/dexamethasone (EPd) significantly improved progression-free survival (PFS) versus pomalidomide/dexamethasone (Pd) in patients with relapsed/refractory multiple myeloma (RRMM) previously treated with lenalidomide and a proteasome inhibitor (PI). Here, we present the final overall survival (OS) results.
Methods: Patients with RRMM who had received ≥ 2 prior lines of therapy, with disease refractory to last therapy and either refractory or relapsed and refractory to lenalidomide and a PI were randomly assigned (1:1) to receive EPd or Pd. The primary end point was PFS per investigator assessment. ORR and OS were secondary end points planned to be tested hierarchically.
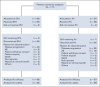
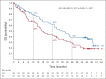
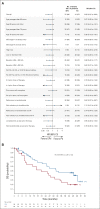
Results: A total of 117 patients were randomly assigned to EPd (n = 60) and Pd (n = 57). Among treated patients (EPd 60, Pd 55), there were 37 (61.7%) deaths in the EPd group and 41 (74.5%) in the Pd group, most commonly because of disease progression (EPd 41.7%, Pd 49.1%). Median (95% CI) OS was significantly improved with EPd (29.8 [22.9 to 45.7] months) versus Pd (17.4 [13.8 to 27.7] months), with a hazard ratio of 0.59 (95% CI, 0.37 to 0.93; P = .0217). OS benefit with EPd was observed in most patient subgroups. The safety profile of EPd was consistent with prior reports with no new safety signals detected.
Conclusion: EPd demonstrated a statistically significant improvement in OS versus Pd in patients with RRMM previously treated with lenalidomide and a PI who had disease refractory to last therapy. In this setting, ELOQUENT-3 is the first randomized study of a triplet regimen incorporating a monoclonal antibody and Pd to improve both PFS and OS significantly.
Conflict of interest statement
No other potential conflicts of interest were reported
Figures
Comment in
-
Overall Survival Analysis of the Use of Elotuzumab in ELOQUENT-3 Trial.J Clin Oncol. 2023 Mar 20;41(9):1788. doi: 10.1200/JCO.22.02098. Epub 2023 Jan 10. J Clin Oncol. 2023. PMID: 36626704 No abstract available.
References
-
- Howlader N, Noone A, Krapcho M, et al. : SEER Cancer Statistics Review, 1975-2018. Bethesda, MD, National Cancer Institute, 2021
-
- Kumar SK, Dimopoulos MA, Kastritis E, et al. : Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: A multicenter IMWG study. Leukemia 31:2443-2448, 2017 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

