Safety and immunogenicity of the first Kazakh inactivated vaccine for COVID-19
- PMID: 35960911
- PMCID: PMC9621059
- DOI: 10.1080/21645515.2022.2087412
Safety and immunogenicity of the first Kazakh inactivated vaccine for COVID-19
Abstract
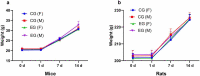
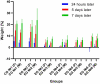
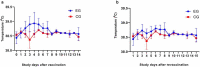
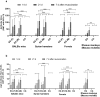
This article describes the results of a preclinical safety and immunogenicity study of QazCovid-in®, the first COVID-19 vaccine developed in Kazakhstan, on BALB/c mice, rats, ferrets, Syrian hamsters and rhesus macaques (Macaca mulatta). The study's safety data suggests that this immunobiological preparation can be technically considered a Class 5 nontoxic vaccine. The series of injections that were made did not produce any adverse effect or any change in the general condition of the model animals' health, while macroscopy and histology studies identified no changes in the internal organs of the BALB/c mice and rats. This study has demonstrated that a double immunization enhances the growth of antibody titers as assessed by the microneutralization assay (MNA) and the enzyme-linked immunosorbent assay (ELISA) in a pre-clinical immunogenicity test on animal models. The best GMT results were assessed in MNA and ELISA 7 days after re-vaccination; however, we noted that GMT antibody results in ELISA were lower than in MNA. A comparative GMT assessment after the first immunization and the re-immunization identified significant differences between model animal groups and a growth of GMT antibodies in all of them; also, differences between the gender groups were statistically significant. Moreover, the most marked MNA immune response to the QazCovid-in® vaccine was seen in the Syrian hamsters, while their SARS-CoV-2-specific antibody activity as assessed with ELISA was the lowest.
Keywords: COVID-19; immunogenicity; preclinical trials; safety; toxicity; vaccine.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
Long-term humoral immunogenicity, safety and protective efficacy of inactivated vaccine against COVID-19 (CoviVac) in preclinical studies.Emerg Microbes Infect. 2021 Dec;10(1):1790-1806. doi: 10.1080/22221751.2021.1971569. Emerg Microbes Infect. 2021. PMID: 34427172 Free PMC article.
-
Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials.JAMA. 2020 Sep 8;324(10):951-960. doi: 10.1001/jama.2020.15543. JAMA. 2020. PMID: 32789505 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): A multicenter study.J Hepatol. 2021 Aug;75(2):439-441. doi: 10.1016/j.jhep.2021.04.026. Epub 2021 Apr 24. J Hepatol. 2021. PMID: 33905793 Free PMC article.
-
Safety and immunogenicity of the SARS-CoV-2 ARCoV mRNA vaccine in Chinese adults: a randomised, double-blind, placebo-controlled, phase 1 trial.Lancet Microbe. 2022 Mar;3(3):e193-e202. doi: 10.1016/S2666-5247(21)00280-9. Epub 2022 Jan 24. Lancet Microbe. 2022. PMID: 35098177 Free PMC article. Clinical Trial.
-
Safety and potency of BIV1-CovIran inactivated vaccine candidate for SARS-CoV-2: A preclinical study.Rev Med Virol. 2022 May;32(3):e2305. doi: 10.1002/rmv.2305. Epub 2021 Oct 26. Rev Med Virol. 2022. PMID: 34699647 Free PMC article. Review.
Cited by
-
Safety and Immunogenicity of the Live Attenuated Vaccine QazCOVID-Live Against Coronavirus Infection COVID-19: Pre-Clinical Study Results.Vaccines (Basel). 2024 Dec 12;12(12):1401. doi: 10.3390/vaccines12121401. Vaccines (Basel). 2024. PMID: 39772061 Free PMC article.
-
Breaking the Barrier: SARS-CoV-2 Infections in Wild and Companion Animals and Their Implications for Public Health.Viruses. 2024 Jun 13;16(6):956. doi: 10.3390/v16060956. Viruses. 2024. PMID: 38932248 Free PMC article. Review.
-
Vaccine research and development capacity in Central and West Asia: A path toward sustainable vaccine R&D programs.Front Public Health. 2023 Mar 2;11:1143790. doi: 10.3389/fpubh.2023.1143790. eCollection 2023. Front Public Health. 2023. PMID: 36935694 Free PMC article.
-
Pre-Clinical Safety and Immunogenicity Study of a Coronavirus Protein-Based Subunit Vaccine for COVID-19.Vaccines (Basel). 2023 Nov 28;11(12):1771. doi: 10.3390/vaccines11121771. Vaccines (Basel). 2023. PMID: 38140175 Free PMC article.
-
Attitude toward vaccination against COVID-19 and acceptance of the national "QazVac" vaccine in the Aktobe city population, West Kazakhstan: A cross-sectional survey.PLoS One. 2024 May 16;19(5):e0303854. doi: 10.1371/journal.pone.0303854. eCollection 2024. PLoS One. 2024. PMID: 38753835 Free PMC article.
References
-
- COVID-19 Map—Johns Hopkins Coronavirus Resource Center. Johns Hopkins coronavirus resource center; 2020. [accessed 2022 Mar 25]. https://coronavirus.jhu.edu/map.html.
-
- World Health Organization . Coronavirus disease (COVID-19). Situation Report – 136; 2020. Jun. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2....
-
- The situation with the coronavirus; [accessed 2022 Mar 25]. https://www.coronavirus2020.kz/.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
