Distinct Reactivity Modes of a Copper Hydride Enabled by an Intramolecular Lewis Acid
- PMID: 35960993
- PMCID: PMC10291504
- DOI: 10.1021/jacs.2c02937
Distinct Reactivity Modes of a Copper Hydride Enabled by an Intramolecular Lewis Acid
Abstract
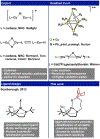
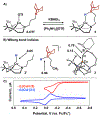
We disclose a 1,4,7-triazacyclononane (TACN) ligand featuring an appended boron Lewis acid. Metalation with Cu(I) affords a series of tetrahedral complexes including a boron-capped cuprous hydride. We demonstrate distinct reactivity modes as a function of chemical oxidation: hydride transfer to CO2 in the copper(I) state and oxidant-induced H2 evolution as well as alkyne reduction.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Lewis Acid-Tethered (cAAC)-Copper Complexes: Reactivity for Hydride Transfer and Catalytic CO2 Hydrogenation.Angew Chem Int Ed Engl. 2024 Oct 21;63(43):e202411099. doi: 10.1002/anie.202411099. Epub 2024 Sep 5. Angew Chem Int Ed Engl. 2024. PMID: 38967599
-
Efficient plasmid DNA cleavage by copper(II) complexes of 1,4,7-triazacyclononane ligands featuring xylyl-linked guanidinium groups.Inorg Chem. 2011 May 16;50(10):4327-39. doi: 10.1021/ic102301n. Epub 2011 Apr 19. Inorg Chem. 2011. PMID: 21504219
-
Phosphodiester cleavage properties of copper(II) complexes of 1,4,7-triazacyclononane ligands bearing single alkyl guanidine pendants.Inorg Chem. 2012 Jan 16;51(2):939-53. doi: 10.1021/ic2019814. Epub 2011 Dec 22. Inorg Chem. 2012. PMID: 22221199
-
Hydrido copper clusters supported by dithiocarbamates: oxidative hydride removal and neutron diffraction analysis of [Cu7(H){S2C(aza-15-crown-5)}6].Inorg Chem. 2012 Jun 18;51(12):6577-91. doi: 10.1021/ic300135w. Epub 2012 Jun 4. Inorg Chem. 2012. PMID: 22663192
-
Copper-biomolecule complexes in the gas phase. The ternary way.Mass Spectrom Rev. 2007 Jul-Aug;26(4):563-82. doi: 10.1002/mas.20137. Mass Spectrom Rev. 2007. PMID: 17474124 Review.
Cited by
-
A Bidentate Ligand Featuring Ditopic Lewis Acids in the Second Sphere for Selective Substrate Capture and Activation.Angew Chem Int Ed Engl. 2023 Mar 20;62(13):e202218907. doi: 10.1002/anie.202218907. Epub 2023 Feb 20. Angew Chem Int Ed Engl. 2023. PMID: 36720708 Free PMC article.
-
Zinc-Catalyzed Hydroboration of Carbon Dioxide Amplified by Borane-Tethered Heteroscorpionate Bis(Pyrazolyl)methane Ligands.Inorg Chem. 2024 May 6;63(18):8244-8256. doi: 10.1021/acs.inorgchem.4c00500. Epub 2024 Apr 24. Inorg Chem. 2024. PMID: 38656156 Free PMC article.
-
Appended Lewis Acids Enable Dioxygen Reactivity and Catalytic Oxidations with Ni(II).J Am Chem Soc. 2024 May 8;146(18):12375-12385. doi: 10.1021/jacs.3c12399. Epub 2024 Apr 25. J Am Chem Soc. 2024. PMID: 38661576 Free PMC article.
-
Borane-functionalized heteroscorpionate copper complexes as catalysts for azide-alkyne cycloaddition.Dalton Trans. 2025 Aug 27. doi: 10.1039/d5dt01595b. Online ahead of print. Dalton Trans. 2025. PMID: 40878191 Free PMC article.
-
A Cooperative Cobalt-Driven System for One-Carbon Extension in the Synthesis of (Z)-Silyl Enol Ethers from Aldehydes: Unlocking Regio- and Stereoselectivity.J Am Chem Soc. 2023 Dec 27;145(51):27922-27932. doi: 10.1021/jacs.3c10491. Epub 2023 Dec 12. J Am Chem Soc. 2023. PMID: 38086018 Free PMC article.
References
-
- Lundgren RJ; Stradiotto M, Key Concepts in Ligand Design. In Ligand Design in Metal Chemistry, 2016; pp 1–14.
-
- Drover MW, A guide to secondary coordination sphere editing. Chem. Soc. Rev 2022, 51 (6), 1861–1880. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

