P450 oxidoreductase regulates barrier maturation by mediating retinoic acid metabolism in a model of the human BBB
- PMID: 35961311
- PMCID: PMC9481905
- DOI: 10.1016/j.stemcr.2022.07.010
P450 oxidoreductase regulates barrier maturation by mediating retinoic acid metabolism in a model of the human BBB
Abstract
The blood-brain barrier (BBB) selectively regulates the entry of molecules into the central nervous system (CNS). A crosstalk between brain microvascular endothelial cells (BMECs) and resident CNS cells promotes the acquisition of functional tight junctions (TJs). Retinoic acid (RA), a key signaling molecule during embryonic development, is used to enhance in vitro BBB models' functional barrier properties. However, its physiological relevance and affected pathways are not fully understood. P450 oxidoreductase (POR) regulates the enzymatic activity of microsomal cytochromes. POR-deficient (PORD) patients display impaired steroid homeostasis and cognitive disabilities. Here, we used both patient-specific POR-deficient and CRISPR-Cas9-mediated POR-depleted induced pluripotent stem cell (iPSC)-derived BMECs (iBMECs) to study the role of POR in the acquisition of functional barrier properties. We demonstrate that POR regulates cellular RA homeostasis and that POR deficiency leads to the accumulation of RA within iBMECs, resulting in the impaired acquisition of TJs and, consequently, to dysfunctional development of barrier properties.
Keywords: BBB; P450 oxidoreductase; POR; blood-brain barrier; cytochrome; disease modeling; iPSCs; metabolism; retinoic acid; tight junctions.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest The authors declare no conflict of interest.
Figures

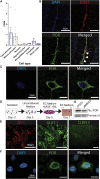
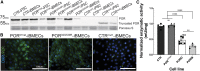
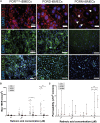


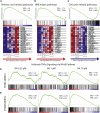
References
-
- Anbalagan S., Gordon L., Blechman J., Matsuoka R.L., Rajamannar P., Wircer E., Biran J., Reuveny A., Leshkowitz D., Stainier D.Y.R., et al. Pituicyte cues regulate the development of permeable neuro-vascular interfaces. Dev. Cell. 2018;47:711–726.e5. doi: 10.1016/j.devcel.2018.10.017. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

