Incidence and Clinical Characteristics of Severe Acute Respiratory Syndrome Coronavirus 2 Infection After Vaccination in Children
- PMID: 35961427
- PMCID: PMC9361575
- DOI: 10.1016/j.jpeds.2022.07.056
Incidence and Clinical Characteristics of Severe Acute Respiratory Syndrome Coronavirus 2 Infection After Vaccination in Children
Abstract
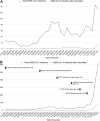
The objective of this single-center cohort study was to characterize the frequency, clinical characteristics, and molecular epidemiology of pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection after vaccination. Between May 15, 2021, and January 1, 2022, 171 children experienced SARS-CoV-2 infection postvaccination, 146 (86%) following the Omicron variant predominance. Outcomes were generally mild and comparable before and after Omicron predominance.
Keywords: COVID-19; SARS-CoV-2; breakthrough; pediatric; vaccine.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures

References
-
- Klein N.P., Stockwell M.S., Demarco M., Gaglani M., Kharbanda A.B., Irving S.A., et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA vaccination in preventing COVID-19-associated emergency department and urgent care encounters and hospitalizations among nonimmunocompromised children and adolescents aged 5-17 years—VISION Network, 10 states, April 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:352–358. - PMC - PubMed
-
- Thompson M.G., Natarajan K., Irving S.A., Rowley E.A., Griggs E.P., Gaglani M., et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:139–145. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

