Availability and costs of medicines for the treatment of tuberculosis in Europe
- PMID: 35961488
- PMCID: PMC9801521
- DOI: 10.1016/j.cmi.2022.07.026
Availability and costs of medicines for the treatment of tuberculosis in Europe
Abstract
Objectives: To evaluate the access to comprehensive diagnostics and novel antituberculosis medicines in European countries.
Methods: We investigated the access to genotypic and phenotypic Mycobacterium tuberculosis drug susceptibility testing and the availability of antituberculosis drugs and calculated the cost of drugs and treatment regimens at major tuberculosis treatment centres in countries of the WHO European region where rates of drug-resistant tuberculosis are the highest among all WHO regions. Results were stratified by middle-income and high-income countries.
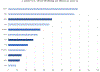
Results: Overall, 43 treatment centres from 43 countries participated in the study. For WHO group A drugs, the frequency of countries with the availability of phenotypic drug susceptibility testing was as follows: (a) 75% (30/40) for levofloxacin, (b) 82% (33/40) for moxifloxacin, (c) 48% (19/40) for bedaquiline, and (d) 72% (29/40) for linezolid. Overall, of the 43 countries, 36 (84%) and 24 (56%) countries had access to bedaquiline and delamanid, respectively, whereas only 6 (14%) countries had access to rifapentine. The treatment of patients with extensively drug-resistant tuberculosis with a regimen including a carbapenem was available only in 17 (40%) of the 43 countries. The median cost of regimens for drug-susceptible tuberculosis, multidrug-resistant/rifampicin-resistant tuberculosis (shorter regimen, including bedaquiline for 6 months), and extensively drug-resistant tuberculosis (including bedaquiline, delamanid, and a carbapenem) were €44 (minimum-maximum, €15-152), €764 (minimum-maximum, €542-15152), and €8709 (minimum-maximum, €7965-11759) in middle-income countries (n = 12) and €280 (minimum-maximum, €78-1084), €29765 (minimum-maximum, €11116-40584), and €217591 (minimum-maximum, €82827-320146) in high-income countries (n = 29), respectively.
Discussion: In countries of the WHO European region, there is a widespread lack of drug susceptibility testing capacity to new and repurposed antituberculosis drugs, lack of access to essential medications in several countries, and a high cost for the treatment of drug-resistant tuberculosis.
Keywords: Antimicrobial resistance; Availability of medicines; Capacity building; Costs; END-TB strategy; MDR-TB; Medicines; Tuberculosis.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Transparency declaration
CLa provided consultation service to INSMED and received speaker’s honoraria from INSMED, GILEAD, and JANSSEN, outside of the scope of this work. The other authors declare that they have no conflicts of interest. CLa is supported by the German Center for Infection Research (DZIF). All other authors have no funding source in the context of this manuscript.
Figures

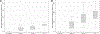

References
-
- World Health Organization. Global tuberculosis report 2021. 2021. Geneva, Switzerland, https://apps.who.int/iris/rest/bitstreams/1379788/retrieve.
-
- World Health Organization. The WHO end TB strategy. 2015. Geneva, Switzerland, https://apps.who.int/iris/rest/bitstreams/1271371/retrieve.
-
- World Health Organization and the European Centre for Disease Prevention and Control. Tuberculosis surveillance and monitoring in Europe 2022 – 2020 data. 2022. Copenhagen.
-
- Dheda K, Gumbo T, Maartens G, Dooley KE, Murray M, Furin J, et al. The Lancet Respiratory Medicine Commission: 2019 update: epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant and incurable tuberculosis. Lancet Respir Med 2019;7:820–6. 10.1016/S2213-2600(19)30263-2. - DOI - PubMed
-
- World Health Organization. WHO consolidated guidelines on tuberculosis. Module 3: diagnosis - rapid diagnostics for tuberculosis detection, 2021 update. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO, https://apps.who.int/iris/rest/bitstreams/1354562/retrieve.

