Secukinumab in enthesitis-related arthritis and juvenile psoriatic arthritis: a randomised, double-blind, placebo-controlled, treatment withdrawal, phase 3 trial
- PMID: 35961761
- PMCID: PMC9811076
- DOI: 10.1136/ard-2022-222849
Secukinumab in enthesitis-related arthritis and juvenile psoriatic arthritis: a randomised, double-blind, placebo-controlled, treatment withdrawal, phase 3 trial
Abstract
Background: Treatment options in patients with enthesitis-related arthritis (ERA) and juvenile psoriatic arthritis (JPsA) are currently limited. This trial aimed to demonstrate the efficacy and safety of secukinumab in patients with active ERA and JPsA with inadequate response to conventional therapy.
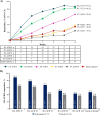
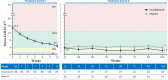
Methods: In this randomised, double-blind, placebo-controlled, treatment-withdrawal, phase 3 trial, biologic-naïve patients (aged 2 to <18 years) with active disease were treated with open-label subcutaneous secukinumab (75/150 mg in patients <50/≥50 kg) in treatment period (TP) 1 up to week 12, and juvenile idiopathic arthritis (JIA) American College of Rheumatology 30 responders at week 12 were randomised 1:1 to secukinumab or placebo up to 100 weeks. Patients who flared in TP2 immediately entered open-label secukinumab TP3 that lasted up to week 104. Primary endpoint was time to disease flare in TP2.
Results: A total of 86 patients (median age, 14 years) entered open-label secukinumab in TP1. In TP2, responders (ERA, 44/52; JPsA, 31/34) received secukinumab or placebo. The study met its primary end point and demonstrated a statistically significant longer time to disease flare in TP2 for ERA and JPsA with secukinumab versus placebo (27% vs 55%, HR, 0.28; 95% CI 0.13 to 0.63; p<0.001). Exposure-adjusted incidence rates (per 100 patient-years (PY), 95% CI) for total patients were 290.7/100 PY (230.2 to 362.3) for adverse events and 8.2/100 PY (4.1 to 14.6) for serious adverse events in the overall JIA population.
Conclusions: Secukinumab demonstrated significantly longer time to disease flare than placebo in children with ERA and JPsA with a consistent safety profile with the adult indications of psoriatic arthritis and axial spondyloarthritis.
Trial registration number: NCT03031782.
Keywords: arthritis, juvenile; arthritis, psoriatic; biological therapy.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: HIB has received grants and consulting fees from Novartis; ICP has received payment honoria for lectures, presentations, speakers bureaus from Novartis; MH has received payment for participation on data safety monitoring board or advisory board from Novartis;GSS has received grants and consulting fees from Novartis; AVR has received consulting fees from Abbvie, Astra Zeneca, Eli Lilly, Novartis, Roche, UCB; payment/honoria for lectures, presentations, speakers bureaus from Abbvie, Eli Lilly, Novartis, Roche, SOBI; payment for participation on data safety monitoring board or advisory board from Eli Lilly; EZ has received grants or contracts from Novartis and Pfizer; payment/honoria for lectures, presentations, speakers bureaus from Abbvie, MSD, Novartis, Pfizer and Roche; has received support for attending meetings and/or travel from Abbvie, MSD, Novartis, Pfizer and Roche; Ruvie Martin, Xuan Zhu, Sarah Whelan and Luminita Pricop are employees and shareholders of Novartis; AM has received consulting fees from Aurinia, Bristol Myers and Squibb, Eli Lilly, EMD, Serono, Janssen, Pfizer and Roche; payment/ honoria for lectures, presentations, speakers bureaus from Aurinia, Bristol Myers and Squibb, Eli Lilly, EMD, Janssen, Pfizer, Roche and Serono; DJL has received grants and consulting fees from Astra Zeneca, Boehringer Ingelheim, GSK, Hoffman LaRoche, Novartis, and UBC; Nicolino Ruperto has received consulting fees from Ablynx, Amgen, Astrazeneca-Medimmune, Aurinia, Bayer, Bristol Myers and Squibb, Cambridge Healthcare Research (CHR), Celegene, Domain therapeutic, Eli Lilly, EMD Serono, GlaxoSmith and Kline, Idorsia, Janssen, Novartis, Pfizer, SOBI and UCB; payment/ honoria for lectures, presentations, speakers bureaus from Eli Lilly, GlaxoSmith and Kline, Pfizer, SOBI and UCB; has received payment for participation on data safety monitoring board or advisory board from Eli Lilly and Pfizer; has received funding from Bristol Myers and Squibb, Eli Lilly, F Hoffmann-La Roche, Novartis, Pfizer and SOBI.
Figures

Comment in
-
Secukinumab in enthesitis-related arthritis and juvenile psoriatic arthritis.Int J Rheum Dis. 2023 Nov;26(11):2119-2121. doi: 10.1111/1756-185X.14845. Epub 2023 Aug 11. Int J Rheum Dis. 2023. PMID: 37563978 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

