Cost-effectiveness of population-wide genomic screening for familial hypercholesterolemia in the United States
- PMID: 35961838
- PMCID: PMC9926472
- DOI: 10.1016/j.jacl.2022.07.014
Cost-effectiveness of population-wide genomic screening for familial hypercholesterolemia in the United States
Abstract
Background: Population genomic screening for familial hypercholesterolemia (FH) in unselected individuals can prevent premature cardiovascular disease.
Objective: To estimate the clinical and economic outcomes of population-wide FH genomic screening versus no genomic screening.
Methods: We developed a decision tree plus 10-state Markov model evaluating the identification of patients with an FH variant, statin treatment status, LDL-C levels, MI, and stroke to compare the costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness of population-wide FH genomic screening. FH variant prevalence (0.4%) was estimated from the Geisinger MyCode Community Health Initiative (MyCode). Genomic test costs were assumed to be $200. Age and sex-based estimates of MI, recurrent MI, stroke, and recurrent stroke were obtained from Framingham risk equations. Additional outcomes independently associated with FH variants were derived from a retrospective analysis of 26,025 participants screened for FH. Sensitivity and threshold analyses were conducted to evaluate model assumptions and uncertainty.
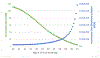
Results: FH screening was most effective at younger ages; screening unselected 20-year-olds lead to 111 QALYs gained per 100,000 individuals screened at an incremental cost of $20 M. The incremental cost-effectiveness ratio (ICER) for 20-year-olds was $181,000 per QALY, and there was a 38% probability of cost-effectiveness at a $100,000 per QALY willingness-to-pay threshold. If genomic testing cost falls to $100, the ICER would be $91,000 per QALY.
Conclusion: Population FH screening is not cost-effective at current willingness to pay thresholds. However, reducing test costs, testing at younger ages, or including FH within broader multiplex screening panels may improve clinical and economic value.
Keywords: Cost-effectiveness analysis; Cost-utility analysis; Familial hypercholesterolemia; Genomic screening; Population screening.
Copyright © 2022. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The authors declare no conflicts of interest. The funders did not have a role in the design of the study, in the collection, analysis, or interpretation of data, in the manuscript writing, or in the decision to publish results.
Figures
References
-
- Benn M, Watts GF, Tybjærg-Hansen A, Nordestgaard BG. Mutations causative of familial hypercholesterolaemia: screening of 98 098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. European heart journal. 2016;37(17):1384–1394. - PubMed
-
- Sjouke B, Kusters DM, Kindt I, et al. Homozygous autosomal dominant hypercholesterolaemia in the Netherlands: prevalence, genotype–phenotype relationship, and clinical outcome. European heart journal. 2015;36(9):560–565. - PubMed
-
- Sturm AC, Knowles JW, Gidding SS, et al. Clinical genetic testing for familial hypercholesterolemia: JACC scientific expert panel. Journal of the American College of Cardiology. 2018;72(6):662–680. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous