Conformational enantiodiscrimination for asymmetric construction of atropisomers
- PMID: 35961985
- PMCID: PMC9374765
- DOI: 10.1038/s41467-022-32432-8
Conformational enantiodiscrimination for asymmetric construction of atropisomers
Abstract
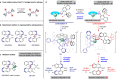
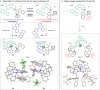
Molecular conformations induced by the rotation about single bonds play a crucial role in chemical transformations. Revealing the relationship between the conformations of chiral catalysts and the enantiodiscrimination is a formidable challenge due to the great difficulty in isolating the conformers. Herein, we report a chiral catalytic system composed of an achiral catalytically active unit and an axially chiral 1,1'-bi-2-naphthol (BINOL) unit which are connected via a C-O single bond. The two conformers of the catalyst induced by the rotation about the C-O bond, are determined via single-crystal X-ray diffraction and found to respectively lead to the formation of highly important axially chiral 1,1'-binaphthyl-2,2'-diamine (BINAM) and 2-amino-2'-hydroxy-1,1'-binaphthyl (NOBIN) derivatives in high yields (up to 98%), with excellent enantioselectivities (up to 98:2 e.r.) and opposite absolute configurations. The results highlight the importance of conformational dynamics of chiral catalysts in asymmetric catalysis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Miyashita A, et al. Synthesis of 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl (BINAP), an atropisomeric chiral bis(triaryl)phosphine, and its use in the rhodium(I)-catalyzed asymmetric hydrogenation of α-(acylamino)acrylic acids. J. Am. Chem. Soc. 1980;102:7932–7934. doi: 10.1021/ja00547a020. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

