Cortical waste clearance in normal and restricted sleep with potential runaway tau buildup in Alzheimer's disease
- PMID: 35961995
- PMCID: PMC9374764
- DOI: 10.1038/s41598-022-15109-6
Cortical waste clearance in normal and restricted sleep with potential runaway tau buildup in Alzheimer's disease
Abstract
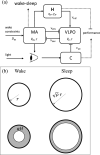
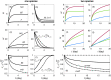
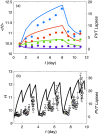
Accumulation of waste in cortical tissue and glymphatic waste clearance via extracellular voids partly drives the sleep-wake cycle and modeling has reproduced much of its dynamics. Here, new modeling incorporates higher void volume and clearance in sleep, multiple waste compounds, and clearance obstruction by waste. This model reproduces normal sleep-wake cycles, sleep deprivation effects, and performance decreases under chronic sleep restriction (CSR). Once fitted to calibration data, it successfully predicts dynamics in further experiments on sleep deprivation, intermittent CSR, and recovery after restricted sleep. The results imply a central role for waste products with lifetimes similar to tau protein. Strong tau buildup is predicted if pathologically enhanced production or impaired clearance occur, with runaway buildup above a critical threshold. Predicted tau accumulation has timescales consistent with the development of Alzheimer's disease. The model unifies a wide sweep of phenomena, clarifying the role of glymphatic clearance and targets for interventions against waste buildup.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- McKenna JT, et al. Nocturnal elevation of extracellular adenosine in the rat basal forebrain. Sleep Res. Online. 2003;5:155–160.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

