Strategies for HIV-1 vaccines that induce broadly neutralizing antibodies
- PMID: 35962033
- PMCID: PMC9372928
- DOI: 10.1038/s41577-022-00753-w
Strategies for HIV-1 vaccines that induce broadly neutralizing antibodies
Erratum in
-
Author Correction: Strategies for HIV-1 vaccines that induce broadly neutralizing antibodies.Nat Rev Immunol. 2023 Apr;23(4):265. doi: 10.1038/s41577-023-00854-0. Nat Rev Immunol. 2023. PMID: 36859597 Free PMC article. No abstract available.
Abstract
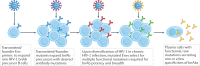
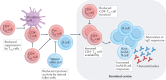
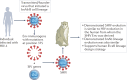
After nearly four decades of research, a safe and effective HIV-1 vaccine remains elusive. There are many reasons why the development of a potent and durable HIV-1 vaccine is challenging, including the extraordinary genetic diversity of HIV-1 and its complex mechanisms of immune evasion. HIV-1 envelope glycoproteins are poorly recognized by the immune system, which means that potent broadly neutralizing antibodies (bnAbs) are only infrequently induced in the setting of HIV-1 infection or through vaccination. Thus, the biology of HIV-1-host interactions necessitates novel strategies for vaccine development to be designed to activate and expand rare bnAb-producing B cell lineages and to select for the acquisition of critical improbable bnAb mutations. Here we discuss strategies for the induction of potent and broad HIV-1 bnAbs and outline the steps that may be necessary for ultimate success.
© 2022. Springer Nature Limited.
Conflict of interest statement
K.O.S. and B.F.H. are inventors on International Patent Application PCT/US2018/020788 submitted by Duke University that covers the composition and use of CH848 HIV-1 Envs for induction of HIV-1 antibodies. K.O.S., K.W. and B.F.H. are inventors on International Patent Application PCT/US2018/03477 submitted by Duke University that covers the composition and use of CH505 HIV-1 Envs for induction of HIV-1 antibodies.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

