Antibody response after first and second BNT162b2 vaccination to predict the need for subsequent injections in nursing home residents
- PMID: 35962053
- PMCID: PMC9373891
- DOI: 10.1038/s41598-022-18041-x
Antibody response after first and second BNT162b2 vaccination to predict the need for subsequent injections in nursing home residents
Abstract
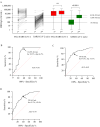
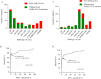
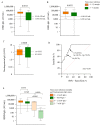
We explored antibody response after first and second BNT162b2 vaccinations, to predict the need for subsequent injections in nursing home (NH) residents. 369 NH residents were tested for IgG against SARS-CoV-2 Receptor-Binding Domain (RBD-IgG) and nucleoprotein-IgG (SARS-CoV-2 IgG II Quant and SARS-CoV-2 IgG Alinity assays, Abbott Diagnostics). In NH residents with prior SARS-CoV-2 infection, the first dose elicited high RBD-IgG levels (≥ 4160 AU/mL) in 99/129 cases (76.9%), with no additional antibody gain after the second dose in 74 cases (74.7%). However, a low RBD-IgG level (< 1050 AU/mL) was observed in 28 (21.7%) residents. The persistence of nucleoprotein-IgG and a longer interval between infection and the first dose were associated with a higher RBD-IgG response (p < 0.0001 and p = 0.0013, respectively). RBD-IgG below 50 AU/mL after the first dose predicted failure to reach the antibody concentration associated with a neutralizing effect after the second dose (≥ 1050 AU/mL). The BNT162b2 vaccine elicited a strong humoral response after the first dose in a majority of NH residents with prior SARS-CoV-2 infection. However, about one quarter of these residents require a second injection. Consideration should be given to immunological monitoring in NH residents to optimize the vaccine response in this vulnerable population.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Canouï-Poitrine F, et al. Magnitude, change over time, demographic characteristics and geographic distribution of excess deaths among nursing home residents during the first wave of COVID-19 in France: A nationwide cohort study. Age Ageing. 2021;50:1473–1481. doi: 10.1093/ageing/afab098. - DOI - PMC - PubMed
-
- Bailly B, et al. BNT162b2 messenger RNA vaccination did not prevent an outbreak of severe acute respiratory syndrome coronavirus 2 variant 501Y.V2 in an elderly nursing home but reduced transmission and disease severity. Clin. Infect. Dis. 2022;74:517–520. doi: 10.1093/cid/ciab446. - DOI - PMC - PubMed
-
- Nanduri S, et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (Delta) variant—National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1163–1166. doi: 10.15585/mmwr.mm7034e3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

