Sensory neuron dysfunction in orthotopic mouse models of colon cancer
- PMID: 35962398
- PMCID: PMC9375288
- DOI: 10.1186/s12974-022-02566-z
Sensory neuron dysfunction in orthotopic mouse models of colon cancer
Abstract
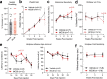
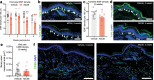
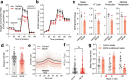
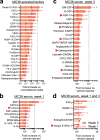
Reports of neurological sequelae related to colon cancer are largely restricted to rare instances of paraneoplastic syndromes, due to autoimmune reactions. Systemic inflammation associated with tumor development influences sensory neuron function in other disease models, though the extent to which this occurs in colorectal cancer is unknown. We induced orthotopic colorectal cancer via orthotopic injection of two colorectal cancer cell lines (MC38 and CT26) in two different mouse strains (C57BL/6 and Balb/c, respectively). Behavioral tests of pain sensitivity and activity did not detect significant alterations in sensory sensitivity or diminished well-being throughout tumor development. However, immunohistochemistry revealed widespread reductions in intraepidermal nerve fiber density in the skin of tumor-bearing mice. Though loss of nerve fiber density was not associated with increased expression of cell injury markers in dorsal root ganglia, lumbar dorsal root ganglia neurons of tumor-bearing animals showed deficits in mitochondrial function. These neurons also had reduced cytosolic calcium levels in live-cell imaging and reduced spontaneous activity in multi-electrode array analysis. Bulk RNA sequencing of DRGs from tumor-bearing mice detected activation of gene expression pathways associated with elevated cytokine and chemokine signaling, including CXCL10. This is consistent with the detection of CXCL10 (and numerous other cytokines, chemokines and growth factors) in MC38 and CT26 cell-conditioned media, and the serum of tumor-bearing mice. Our study demonstrates in a pre-clinical setting that colon cancer is associated with latent sensory neuron dysfunction and implicates cytokine/chemokine signaling in this process. These findings may have implications for determining risk factors and treatment responsiveness related to neuropathy in colorectal cancer.
Keywords: Colon cancer; DRG neuron; Neuropathic pain; Neuropathy; Paraneoplastic neuropathy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Naghavi M, Wang H, Lozano R, Davis A, Liang X, Zhou M, et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. - DOI - PMC - PubMed
-
- Bouchenaki H, Danigo A, Sturtz F, Hajj R, Magy L, Demiot C. An overview of ongoing clinical trials assessing pharmacological therapeutic strategies to manage chemotherapy-induced peripheral neuropathy, based on preclinical studies in rodent models. Fundam Clin Pharmacol. 2021 doi: 10.1111/fcp.12617. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

