Lactobacillus rhamnosus GG ameliorates DON-induced intestinal damage depending on the enrichment of beneficial bacteria in weaned piglets
- PMID: 35962456
- PMCID: PMC9375241
- DOI: 10.1186/s40104-022-00737-9
Lactobacillus rhamnosus GG ameliorates DON-induced intestinal damage depending on the enrichment of beneficial bacteria in weaned piglets
Abstract
Background: Deoxynivalenol (DON) is one of the most common environmental pollutants that induces intestinal inflammation and microbiota dysbiosis. Lactobacillus rhamnosus GG (LGG) is a probiotic that not only has anti-inflammatory effects, but also shows protective effect on the intestinal barrier. However, it is still unknown whether LGG exerts beneficial effects against DON-induced intestinal damage in piglets. In this work, a total of 36 weaned piglets were randomized to one of four treatment groups for 21 d. The treatment groups were CON (basal diet); LGG (basal diet supplemented with 1.77 × 1011 CFU/kg LGG); DON (DON-contaminated diet) and LGG + DON (DON-contaminated diet supplemented with 1.77 × 1011 CFU/kg LGG).
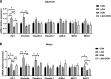
Result: Supplementation of LGG can enhance growth performance of piglets exposed to DON by improving intestinal barrier function. LGG has a mitigating effect on intestinal inflammation induced by DON exposure, largely through repression of the TLR4/NF-κB signaling pathway. Furthermore, supplementation of LGG increased the relative abundances of beneficial bacteria (e.g., Collinsella, Lactobacillus, Ruminococcus_torques_group and Anaerofustis), and decreased the relative abundances of harmful bacteria (e.g., Parabacteroides and Ruminiclostridium_6), and also promoted the production of SCFAs.
Conclusions: LGG ameliorates DON-induced intestinal damage, which may provide theoretical support for the application of LGG to alleviate the adverse effects induced by DON exposure.
Keywords: Deoxynivalenol; Gut microbiota; Intestinal inflammation; Lactobacillus rhamnosus GG; Piglets.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



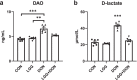






References
Grants and funding
LinkOut - more resources
Full Text Sources

