Pronounced antibody elevation after SARS-CoV-2 BNT162b2 mRNA booster vaccination in nursing home residents
- PMID: 35962568
- PMCID: PMC9530588
- DOI: 10.1111/irv.13030
Pronounced antibody elevation after SARS-CoV-2 BNT162b2 mRNA booster vaccination in nursing home residents
Abstract
Background: Infection control during COVID-19 outbreaks in nursing facilities is a critical public health issue. Antibody responses before and after the third (booster) dose of SARS-CoV-2 vaccination in nursing home residents have not been fully characterized.
Methods: This study included 117 individuals: 54 nursing home residents (mean age, 83.8 years; 39 SARS-CoV-2-naive and 15 previously infected) and 63 healthcare workers (mean age, 45.8 years; 32 SARS-CoV-2-naive and 31 previously infected). Anti-spike (receptor-binding domain [RBD]) and anti-nucleocapsid antibody responses to BNT162b2 mRNA vaccination and their related factors were evaluated using pre- (shortly and 6 months after the second dose) and post-booster vaccination samples.
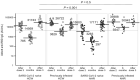
Results: The median anti-spike (RBD) IgG level in SARS-CoV-2-naive residents 6 months after the second dose was the lowest among the four groups, with a decreasing rate of over 90%. The median rate of increase before and after the third dose in SARS-CoV-2-naive residents was significantly higher than that in SARS-CoV-2-naive healthcare workers (64.1- vs. 37.0-fold, P = 0.003), with the highest level among the groups. The IgG ratio of SARS-CoV-2-naive residents to healthcare workers after the second and third doses changed from one-fifth (20%) to one-half (50%). The rate of increase after the third dose in previously infected individuals was three- to fourfold, regardless of residents or healthcare workers.
Conclusions: Advanced aged nursing home residents, poor responders in the initial SARS-CoV-2 vaccine series, could obtain sufficient antibody responses with the additional booster dose, despite more than 6 months after the second.
Keywords: COVID-19; SARS-CoV-2; antibody response; booster vaccination; nursing home residents.
© 2022 The Authors. Influenza and Other Respiratory Viruses published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures

References
-
- Nanduri S, Pilishvili T, Derado G, et al. Effectiveness of Pfizer‐BioNTech and Moderna vaccines in preventing SARS‐CoV‐2 infection among nursing home residents before and during widespread circulation of the SARS‐CoV‐2 B.1.617.2 (Delta) variant—National Healthcare Safety Network, March 1‐August 1, 2021. Morb Mortal Wkly Rep. 2021;70(34):1163‐1166. doi: 10.15585/mmwr.mm7034e3 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

