A companion to the preclinical common data elements and case report forms for in vivo rodent neuroimaging: A report of the TASK3-WG3 Neuroimaging Working Group of the ILAE/AES Joint Translational Task Force
- PMID: 35962745
- PMCID: PMC12375987
- DOI: 10.1002/epi4.12643
A companion to the preclinical common data elements and case report forms for in vivo rodent neuroimaging: A report of the TASK3-WG3 Neuroimaging Working Group of the ILAE/AES Joint Translational Task Force
Abstract
The International League Against Epilepsy/American Epilepsy Society (ILAE/AES) Joint Translational Task Force established the TASK3 working groups to create common data elements (CDEs) for various aspects of preclinical epilepsy research studies, which could help improve the standardization of experimental designs. In this article, we discuss CDEs for neuroimaging data that are collected in rodent models of epilepsy, with a focus on adult rats and mice. We provide detailed CDE tables and case report forms (CRFs), and with this companion manuscript, we discuss the methodologies for several imaging modalities and the parameters that can be collected.
Keywords: epilepsy; magnetic resonance imaging; magnetic resonance spectroscopy; mouse; positron emission tomography; rat; single photon emission computed tomography.
© 2022 The Authors. Epilepsia Open published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
Alon Friedman is Associate Editor for
Figures









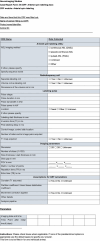








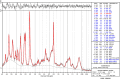



References
-
- Harte‐Hargrove LC, French JA, Pitkanen A, Galanopoulou AS, Whittemore V, Scharfman HE. Common data elements for preclinical epilepsy research: standards for data collection and reporting. A TASK3 report of the AES/ILAE Translational Task Force of the ILAE. Epilepsia. 2017;58(Suppl 4):78–86. - PMC - PubMed
-
- Harte‐Hargrove LC, Galanopoulou AS, French JA, Pitkanen A, Whittemore V, Scharfman HE. Common data elements (CDEs) for preclinical epilepsy research: introduction to CDEs and description of core CDEs. A TASK3 report of the ILAE/AES joint translational task force. Epilepsia Open. 2018;3:13–23. - PMC - PubMed
-
- Barker‐Haliski M, Harte‐Hargrove LC, Ravizza T, Smolders I, Xiao B, Brandt C, et al. A companion to the preclinical common data elements for pharmacologic studies in animal models of seizures and epilepsy. A Report of the TASK3 Pharmacology Working Group of the ILAE/AES Joint Translational Task Force. Epilepsia Open. 2018;3:53–68. - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

