Effects of Acid-Reducing Agents on the Pharmacokinetics of Lazertinib in Patients with EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer
- PMID: 35962934
- PMCID: PMC9464755
- DOI: 10.1007/s12325-022-02286-z
Effects of Acid-Reducing Agents on the Pharmacokinetics of Lazertinib in Patients with EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer
Abstract
Introduction: Lazertinib is an irreversible, mutant-selective epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI). Co-administration of TKIs with acid-reducing agents (ARAs) can lead to potential drug-drug interactions, which decreases solubility and absorption of TKIs and is ultimately associated with reduced efficacy of TKIs. This retrospective analysis evaluated the effect of ARAs on the pharmacokinetics of lazertinib using data obtained from patients with advanced EGFR mutation-positive non-small-cell lung cancer.
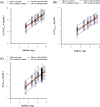
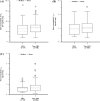
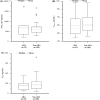
Methods: In a total of 234 patients with lazertinib pharmacokinetics observed at steady state, dose-normalized (DN) area under the concentration-time curve (AUCss), maximum concentration (Cmax,ss), and/or trough concentration on day 15 (CD15) were compared between a group receiving ARA concomitantly for at least 4 days (ARA group) and another group not receiving ARA (non-ARA group) in a dose-proportional range. Additionally, a comparison of pharmacokinetic parameters at a therapeutic dose of 240 mg once daily was evaluated.
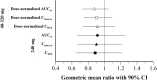
Results: Geometric mean ratios (GMRs) with 90% confidence intervals (CIs) of ARA group to non-ARA group for DNAUCss, DNCmax,ss, and DNCD15 at 40 mg to 320 mg once daily showing the dose proportionality were 0.8743 (0.7285-1.0493), 0.9035 (0.7482-1.0910), and 0.9126 (0.7364-1.1311), respectively. GMRs with 90% CIs for AUCss, Cmax,ss, and CD15 at 240 mg were 0.9136 (0.6637-1.2576), 0.9012 (0.6703-1.2116), and 0.8850 (0.6463-1.2118), respectively.
Conclusion: All pharmacokinetic parameters were not significantly different between the two groups (p values > 0.05), indicating that co-administered ARAs did not significantly affect the steady state pharmacokinetics of lazertinib. Therefore, no dose adjustment of lazertinib is required in patients receiving concomitant ARAs.
Gov identifiers: NCT03046992, NCT04075396.
Keywords: Acid-reducing agent; Drug–drug interactions; Lazertinib; Pharmacokinetics; Tyrosine kinase inhibitor.
© 2022. The Author(s).
Figures
References
-
- Hong MH, Lee IY, Koh JS, et al. P3.02b–119 YH25448, a highly selective 3rd generation EGFR TKI, exhibits superior survival over osimertinib in animal model with brain metastases from NSCLC: Topic: EGFR RES. J Thorac Oncol. 2017;12(1):S1265–S1266. doi: 10.1016/j.jtho.2016.11.1787. - DOI
-
- Ministry of Food and Drug Safety Republic of Korea, LECLAZA (lazertinib): prescribing information. 2022.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

