Aspirin promotes RSL3-induced ferroptosis by suppressing mTOR/SREBP-1/SCD1-mediated lipogenesis in PIK3CA-mutant colorectal cancer
- PMID: 35963119
- PMCID: PMC9389304
- DOI: 10.1016/j.redox.2022.102426
Aspirin promotes RSL3-induced ferroptosis by suppressing mTOR/SREBP-1/SCD1-mediated lipogenesis in PIK3CA-mutant colorectal cancer
Erratum in
-
Corrigendum to "Aspirin promotes RSL3-induced ferroptosis by suppressing mTOR/SREBP-1/SCD1-mediated lipogenesis in PIK3CA-mutatnt colorectal cancer" [Redox Biol. 55 (2022) 102426].Redox Biol. 2025 Mar;80:103533. doi: 10.1016/j.redox.2025.103533. Epub 2025 Feb 15. Redox Biol. 2025. PMID: 39955259 Free PMC article. No abstract available.
Abstract
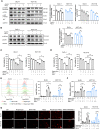
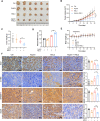
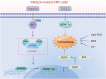
Ferroptosis, a new form of regulated cell death triggered by the iron-dependent peroxidation of phospholipids, is associated with cellular metabolism, redox homeostasis, and various signaling pathways related to cancer. Aspirin is a widely used non-steroidal anti-inflammatory drug (NSAID) and has been reported to show therapeutic benefit in cancers harboring oncogenic PIK3CA, which encodes the catalytic p110α subunit of phosphoinositide 3-kinase (PI3K). In this study, we found that aspirin sensitized cancer cells harboring oncogenic activation of PIK3CA to ferroptosis induction. Mechanistically, aspirin inhibited protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling, suppressed downstream sterol regulatory element-binding protein 1 (SREBP-1) expression, and attenuated stearoyl-CoA desaturase-1 (SCD1)-mediated lipogenesis of monounsaturated fatty acids, thus promoting RSL3-induced ferroptosis in colorectal cancer (CRC) cells. Moreover, genetic ablation of SREBP-1 or SCD1 conferred cancer cells greater sensitivity to ferroptosis induction. Conversely, ectopic expression of SREBP-1 or SCD1 restored ferroptosis resistance in CRC cells and abolished the effect of aspirin on RSL3-induced cytotoxicity. Additionally, the synergistic effects of aspirin and RSL3 were confirmed in a xenograft mouse model. The combined use of aspirin and RSL3 resulted in significant tumor suppression. Our work demonstrated that aspirin enhanced the cytotoxic effect of RSL3 in PIK3CA-mutant cancers, and the combination of aspirin and ferroptosis inducer displayed promising therapeutic effects in cancer treatment.
Keywords: Aspirin; Colorectal cancer; Ferroptosis; SCD1; mTOR.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to disclose. Fig. 7 is created using Figdraw (
Figures




Similar articles
-
Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis.Proc Natl Acad Sci U S A. 2020 Dec 8;117(49):31189-31197. doi: 10.1073/pnas.2017152117. Epub 2020 Nov 23. Proc Natl Acad Sci U S A. 2020. PMID: 33229547 Free PMC article.
-
Long non-coding RNA KB-1460A1.5 promotes ferroptosis by inhibiting mTOR/SREBP-1/SCD1-mediated polyunsaturated fatty acid desaturation in glioma.Carcinogenesis. 2024 Jul 8;45(7):487-499. doi: 10.1093/carcin/bgae016. Carcinogenesis. 2024. PMID: 38422369
-
Corrigendum to "Aspirin promotes RSL3-induced ferroptosis by suppressing mTOR/SREBP-1/SCD1-mediated lipogenesis in PIK3CA-mutatnt colorectal cancer" [Redox Biol. 55 (2022) 102426].Redox Biol. 2025 Mar;80:103533. doi: 10.1016/j.redox.2025.103533. Epub 2025 Feb 15. Redox Biol. 2025. PMID: 39955259 Free PMC article. No abstract available.
-
Stearoyl coenzyme A desaturase-1: multitasker in cancer, metabolism, and ferroptosis.Trends Cancer. 2023 Jun;9(6):480-489. doi: 10.1016/j.trecan.2023.03.003. Epub 2023 Apr 5. Trends Cancer. 2023. PMID: 37029018 Review.
-
Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis.Curr Top Microbiol Immunol. 2017;403:143-170. doi: 10.1007/82_2016_508. Curr Top Microbiol Immunol. 2017. PMID: 28204974 Review.
Cited by
-
The essential roles of lncRNAs/PI3K/AKT axis in gastrointestinal tumors.Front Cell Dev Biol. 2024 Aug 5;12:1442193. doi: 10.3389/fcell.2024.1442193. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39161590 Free PMC article. Review.
-
Circular RNA RHBDD1 regulates tumorigenicity and ferroptosis in colorectal cancer by mediating the ELAVL1/SCD mRNA interaction.Cancer Gene Ther. 2024 Feb;31(2):237-249. doi: 10.1038/s41417-023-00698-9. Epub 2023 Dec 11. Cancer Gene Ther. 2024. PMID: 38072968
-
Role of ferroptosis in colorectal cancer.World J Gastrointest Oncol. 2023 Feb 15;15(2):225-239. doi: 10.4251/wjgo.v15.i2.225. World J Gastrointest Oncol. 2023. PMID: 36908317 Free PMC article. Review.
-
Ferroptosis Inducers Kill Mesenchymal Stem Cells Affected by Neuroblastoma.Cancers (Basel). 2023 Feb 18;15(4):1301. doi: 10.3390/cancers15041301. Cancers (Basel). 2023. PMID: 36831642 Free PMC article.
-
LPCAT1-mediated membrane phospholipid remodelling promotes ferroptosis evasion and tumour growth.Nat Cell Biol. 2024 May;26(5):811-824. doi: 10.1038/s41556-024-01405-y. Epub 2024 Apr 26. Nat Cell Biol. 2024. PMID: 38671262
References
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca - Cancer J. Clin. 2021;71(3):209–249. - PubMed
-
- Dekker E., Tanis P.J., Vleugels J.L.A., Kasi P.M., Wallace M.B. Colorectal cancer. Lancet. 2019;394(10207):1467–1480. - PubMed
-
- Schreuders E.H., Ruco A., Rabeneck L., Schoen R.E., Sung J.J., Young G.P., et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64(10):1637–1649. - PubMed
-
- Ladabaum U., Dominitz J.A., Kahi C., Schoen R.E. Strategies for colorectal cancer screening. Gastroenterology. 2020;158(2):418–432. - PubMed
-
- Keum N., Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019;16(12):713–732. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

