Phosphatidic acid inhibits inositol synthesis by inducing nuclear translocation of kinase IP6K1 and repression of myo-inositol-3-P synthase
- PMID: 35963434
- PMCID: PMC9478396
- DOI: 10.1016/j.jbc.2022.102363
Phosphatidic acid inhibits inositol synthesis by inducing nuclear translocation of kinase IP6K1 and repression of myo-inositol-3-P synthase
Abstract
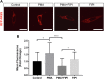
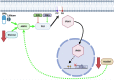
Inositol is an essential metabolite that serves as a precursor for structural and signaling molecules. Although perturbation of inositol homeostasis has been implicated in numerous human disorders, surprisingly little is known about how inositol levels are regulated in mammalian cells. A recent study in mouse embryonic fibroblasts demonstrated that nuclear translocation of inositol hexakisphosphate kinase 1 (IP6K1) mediates repression of myo-inositol-3-P synthase (MIPS), the rate-limiting inositol biosynthetic enzyme. Binding of IP6K1 to phosphatidic acid (PA) is required for this repression. Here, we elucidate the role of PA in IP6K1 repression. Our results indicate that increasing PA levels through pharmacological stimulation of phospholipase D (PLD) or direct supplementation of 18:1 PA induces nuclear translocation of IP6K1 and represses expression of the MIPS protein. We found that this effect was specific to PA synthesized in the plasma membrane, as endoplasmic reticulum-derived PA did not induce IP6K1 translocation. Furthermore, we determined that PLD-mediated PA synthesis can be stimulated by the master metabolic regulator 5' AMP-activated protein kinase (AMPK). We show that activation of AMPK by glucose deprivation or by treatment with the mood-stabilizing drugs valproate or lithium recapitulated IP6K1 nuclear translocation and decreased MIPS expression. This study demonstrates for the first time that modulation of PA levels through the AMPK-PLD pathway regulates IP6K1-mediated repression of MIPS.
Keywords: AMPK; IP6K1; MIPS; glucose; inositol; inositol phosphate; lithium; phosphatidic acid; phospholipase D; valproate.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures





References
-
- Case K.C., Salsaa M., Yu W., Greenberg M.L. Regulation of inositol biosynthesis: balancing health and pathophysiology. Handb. Exp. Pharmacol. 2020;259:221–260. - PubMed
-
- Matile P. Inositol deficiency resulting in death: an explanation of its occurrence in Neurospora crassa. Science. 1966;151:86–88. - PubMed
-
- Eagle H., Oyama V.I., Levy M., Freeman A.E. Myo-Inositol as an essential growth factor for normal and malignant human cells in tissue culture. J. Biol. Chem. 1957;226:191–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

