A lipid nanoparticle platform for mRNA delivery through repurposing of cationic amphiphilic drugs
- PMID: 35963467
- PMCID: PMC9401634
- DOI: 10.1016/j.jconrel.2022.08.009
A lipid nanoparticle platform for mRNA delivery through repurposing of cationic amphiphilic drugs
Abstract
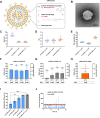
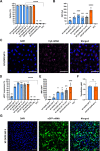
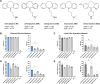
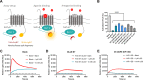
Since the recent clinical approval of siRNA-based drugs and COVID-19 mRNA vaccines, the potential of RNA therapeutics for patient healthcare has become widely accepted. Lipid nanoparticles (LNPs) are currently the most advanced nanocarriers for RNA packaging and delivery. Nevertheless, the intracellular delivery efficiency of state-of-the-art LNPs remains relatively low and safety and immunogenicity concerns with synthetic lipid components persist, altogether rationalizing the exploration of alternative LNP compositions. In addition, there is an interest in exploiting LNP technology for simultaneous encapsulation of small molecule drugs and RNA in a single nanocarrier. Here, we describe how well-known tricyclic cationic amphiphilic drugs (CADs) can be repurposed as both structural and functional components of lipid-based NPs for mRNA formulation, further referred to as CADosomes. We demonstrate that selected CADs, such as tricyclic antidepressants and antihistamines, self-assemble with the widely-used helper lipid DOPE to form cationic lipid vesicles for subsequent mRNA complexation and delivery, without the need for prior lipophilic derivatization. Selected CADosomes enabled efficient mRNA delivery in various in vitro cell models, including easy-to-transfect cancer cells (e.g. human cervical carcinoma HeLa cell line) as well as hard-to-transfect primary cells (e.g. primary bovine corneal epithelial cells), outperforming commercially available cationic liposomes and state-of-the-art LNPs. In addition, using the antidepressant nortriptyline as a model compound, we show that CADs can maintain their pharmacological activity upon CADosome incorporation. Furthermore, in vivo proof-of-concept was obtained, demonstrating CADosome-mediated mRNA delivery in the corneal epithelial cells of rabbit eyes, which could pave the way for future applications in ophthalmology. Based on our results, the co-formulation of CADs, helper lipids and mRNA into lipid-based nanocarriers is proposed as a versatile and straightforward approach for the rational development of drug combination therapies.
Keywords: Cationic amphiphilic drugs; Cellular delivery; Drug repurposing; Lipid nanoparticles; Messenger RNA; Nanomedicine; RNA therapeutics.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare no competing financial interest.
Figures



References
-
- Kanasty R., Dorkin J.R., Vegas A., Anderson D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013;12:967–977. - PubMed
-
- DeWeerdt S. RNA therapies explained. Nature. 2019;574:S2–S3.
-
- Setten R.L., Rossi J.J., Han S.P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019;18:421–446. - PubMed
-
- Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics-developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

