Electrophysiological and Clinical Predictors of Methylphenidate, Guanfacine, and Combined Treatment Outcomes in Children With Attention-Deficit/Hyperactivity Disorder
- PMID: 35963559
- PMCID: PMC9911553
- DOI: 10.1016/j.jaac.2022.08.001
Electrophysiological and Clinical Predictors of Methylphenidate, Guanfacine, and Combined Treatment Outcomes in Children With Attention-Deficit/Hyperactivity Disorder
Abstract
Objective: The combination of d-methylphenidate and guanfacine (an α-2A agonist) has emerged as a potential alternative to either monotherapy in children with attention-deficit/hyperactivity disorder (ADHD), but it is unclear what predicts response to these treatments. This study is the first to investigate pretreatment clinical and electroencephalography (EEG) profiles as predictors of treatment outcome in children randomized to these different medications.
Method: A total of 181 children with ADHD (aged 7-14 years; 123 boys) completed an 8-week randomized, double-blind, comparative study with d-methylphenidate, guanfacine, or combined treatments. Pretreatment assessments included ratings on ADHD, anxiety, and oppositional behavior. EEG activity from cortical sources localized within midfrontal and midoccipital regions was measured during a spatial working memory task with encoding, maintenance, and retrieval phases. Analyses tested whether pretreatment clinical and EEG measures predicted treatment-related change in ADHD severity.
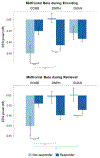
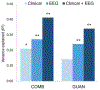
Results: Higher pretreatment hyperactivity-impulsivity and oppositional symptoms and lower anxiety predicted greater ADHD improvements across all medication groups. Pretreatment event-related midfrontal beta power predicted treatment outcome with combined and monotherapy treatments, albeit in different directions. Weaker beta modulations predicted improvements with combined treatment, whereas stronger modulation during encoding and retrieval predicted improvements with d-methylphenidate and guanfacine, respectively. A multivariate model including EEG and clinical measures explained twice as much variance in ADHD improvement with guanfacine and combined treatment (R2= 0.34-0.41) as clinical measures alone (R2 = 0.14-.21).
Conclusion: We identified treatment-specific and shared predictors of response to different pharmacotherapies in children with ADHD. If replicated, these findings would suggest that aggregating information from clinical and brain measures may aid personalized treatment decisions in ADHD.
Clinical trial registration information: Single Versus Combination Medication Treatment for Children With Attention Deficit Hyperactivity Disorder; https://clinicaltrials.gov; NCT00429273.
Keywords: attention-deficit/hyperactivity disorder; electroencephalography; guanfacine; methylphenidate; predictor.
Copyright © 2022 American Academy of Child and Adolescent Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Cortese S, Adamo N, Del Giovane C, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727–738. doi: 10.1016/S2215-0366(18)30269-4 - DOI - PMC - PubMed
-
- McCracken JT, McGough JJ, Loo SK, et al. Combined Stimulant and Guanfacine Administration in Attention-Deficit/Hyperactivity Disorder: A Controlled, Comparative Study. Journal of the American Academy of Child & Adolescent Psychiatry. 2016;55(8):657–666.e1. doi: 10.1016/j.jaac.2016.05.015 - DOI - PMC - PubMed
-
- Connor DF, Findling RL, Kollins SH, et al. Effects of guanfacine extended release on oppositional symptoms in children aged 6-12 years with attention-deficit hyperactivity disorder and oppositional symptoms: a randomized, double-blind, placebo-controlled trial. CNS Drugs. 2010;24(9):755–768. doi: 10.2165/11537790-000000000-00000 - DOI - PubMed

