Lipoprotein Assessment in the twenty-first Century
- PMID: 35963624
- PMCID: PMC9382697
- DOI: 10.1016/j.ecl.2022.02.009
Lipoprotein Assessment in the twenty-first Century
Abstract
Based on decades of both basic science and epidemiologic research, there is overwhelming evidence for the causal relationship between high levels of cholesterol, especially low-density lipoprotein cholesterol and cardiovascular disease. Risk evaluation and monitoring the response to lipid-lowering therapies are heavily dependent on the accurate assessment of plasma lipoproteins in the clinical laboratory. This article provides an update of lipoprotein metabolism as it relates to atherosclerosis and how diagnostic measures of lipids and lipoproteins can serve as markers of cardiovascular risk, with a focus on recent advances in cardiovascular risk marker testing.
Keywords: Advanced lipoprotein testing; Cardiovascular risk assessment; HDL functionality; LDL-C calculation; Lipoprotein (a); Lipoprotein diagnostic assays; Non-HDL cholesterol; Recommendations for lipoprotein assessment.
Published by Elsevier Inc.
Figures
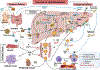



References
-
- Feingold KR, Introduction to Lipids and Lipoproteins, in Endotext, Feingold KR, et al., Editors. 2000: South Dartmouth (MA).
-
- Gennemark P, et al., An oral antisense oligonucleotide for PCSK9 inhibition. Sci Transl Med, 2021. 13(593). - PubMed
-
- Pettersen D and Fjellstrom O, Small molecule modulators of PCSK9 - A literature and patent overview. Bioorg Med Chem Lett, 2018. 28(7): p. 1155–1160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

