Three-dimensional culture models of human endometrium for studying trophoblast-endometrium interaction during implantation
- PMID: 35964080
- PMCID: PMC9375428
- DOI: 10.1186/s12958-022-00973-8
Three-dimensional culture models of human endometrium for studying trophoblast-endometrium interaction during implantation
Abstract
During implantation, a symphony of interaction between the trophoblast originated from the trophectoderm of the implanting blastocyst and the endometrium leads to a successful pregnancy. Defective interaction between the trophoblast and endometrium often results in implantation failure, pregnancy loss, and a number of pregnancy complications. Owing to ethical concerns of using in vivo approaches to study human embryo implantation, various in vitro culture models of endometrium were established in the past decade ranging from two-dimensional cell-based to three-dimensional extracellular matrix (ECM)/tissue-based culture systems. Advanced organoid systems have also been established for recapitulation of different cellular components of the maternal-fetal interface, including the endometrial glandular organoids, trophoblast organoids and blastoids. However, there is no single ideal model to study the whole implantation process leaving more research to be done pursuing the establishment of a comprehensive in vitro model that can recapitulate the biology of trophoblast-endometrium interaction during early pregnancy. This would allow us to have better understanding of the physiological and pathological process of trophoblast-endometrium interaction during implantation.
Keywords: 3D models; Endometrium; Implantation; Organoid; Placentation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


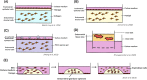

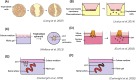

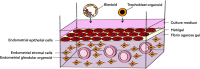
References
Publication types
MeSH terms
Grants and funding
- HKUSZH201902015/High Level-Hospital Program, Health Commission of Guangdong Province, China
- HKUSZH201902015/High Level-Hospital Program, Health Commission of Guangdong Province, China
- HKUSZH201902015/High Level-Hospital Program, Health Commission of Guangdong Province, China
- HKUSZH201902015/High Level-Hospital Program, Health Commission of Guangdong Province, China
- HKUSZH201902015/High Level-Hospital Program, Health Commission of Guangdong Province, China
- HKUSZH201902015/High Level-Hospital Program, Health Commission of Guangdong Province, China
- SZXK2020089/HKU-SZH Fund for Shenzhen Key Medical Discipline
- SZXK2020089/HKU-SZH Fund for Shenzhen Key Medical Discipline
- SZXK2020089/HKU-SZH Fund for Shenzhen Key Medical Discipline
- SZXK2020089/HKU-SZH Fund for Shenzhen Key Medical Discipline
- SZXK2020089/HKU-SZH Fund for Shenzhen Key Medical Discipline
- SZXK2020089/HKU-SZH Fund for Shenzhen Key Medical Discipline
- 17115619/Hong Kong Research Grant Council Grant
- 17115619/Hong Kong Research Grant Council Grant
- 17115619/Hong Kong Research Grant Council Grant
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

