Targeting purine metabolism in ovarian cancer
- PMID: 35964092
- PMCID: PMC9375293
- DOI: 10.1186/s13048-022-01022-z
Targeting purine metabolism in ovarian cancer
Abstract
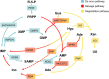
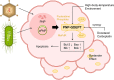
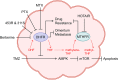
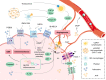
Purine, an abundant substrate in organisms, is a critical raw material for cell proliferation and an important factor for immune regulation. The purine de novo pathway and salvage pathway are tightly regulated by multiple enzymes, and dysfunction in these enzymes leads to excessive cell proliferation and immune imbalance that result in tumor progression. Maintaining the homeostasis of purine pools is an effective way to control cell growth and tumor evolution, and exploiting purine metabolism to suppress tumors suggests interesting directions for future research. In this review, we describe the process of purine metabolism and summarize the role and potential therapeutic effects of the major purine-metabolizing enzymes in ovarian cancer, including CD39, CD73, adenosine deaminase, adenylate kinase, hypoxanthine guanine phosphoribosyltransferase, inosine monophosphate dehydrogenase, purine nucleoside phosphorylase, dihydrofolate reductase and 5,10-methylenetetrahydrofolate reductase. Purinergic signaling is also described. We then provide an overview of the application of purine antimetabolites, comprising 6-thioguanine, 6-mercaptopurine, methotrexate, fludarabine and clopidogrel. Finally, we discuss the current challenges and future opportunities for targeting purine metabolism in the treatment-relevant cellular mechanisms of ovarian cancer.
Keywords: Antimetabolites; Metabolizing enzyme; Ovarian cancer; Purine metabolism; Purinergic signaling.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

