Study protocol and methods for Easing Pelvic Pain Interventions Clinical Research Program (EPPIC): a randomized clinical trial of brief, low-intensity, transdiagnostic cognitive behavioral therapy vs education/support for urologic chronic pelvic pain syndrome (UCPPS)
- PMID: 35964133
- PMCID: PMC9375413
- DOI: 10.1186/s13063-022-06554-9
Study protocol and methods for Easing Pelvic Pain Interventions Clinical Research Program (EPPIC): a randomized clinical trial of brief, low-intensity, transdiagnostic cognitive behavioral therapy vs education/support for urologic chronic pelvic pain syndrome (UCPPS)
Abstract
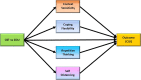
Background: Urologic chronic pelvic pain syndrome (UCPPS) encompasses several common, costly, diagnoses including interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome that are poorly understood and inadequately treated with conventional medical therapies. Behavioral strategies, recommended as a first-line treatment for managing symptoms, are largely inaccessible, time and labor intensive, and technically complex. The Easing Pelvic Pain Interventions Clinical Research Program (EPPIC) is a clinical trial examining the efficacy of low-intensity cognitive behavioral therapy (Minimal Contact CBT or MC-CBT) for UCPPS and its durability 3 and 6 months post treatment. Additional aims include characterizing the operative processes (e.g., cognitive distancing, context sensitivity, coping flexibility, repetitive negative thought) that drive MC-CBT-induced symptom relief and pre-treatment patient variables that moderate differential response.
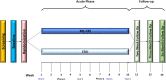
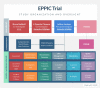
Methods: UCPPS patients (240) ages 18-70 years, any gender, ethnicity, and race, will be randomized to 4-session MC-CBT or a credible, non-specific education comparator (EDU) that controls for the generic effects from simply going to treatment. Efficacy assessments will be administered at pre-treatment, 2 weeks, and 3 and 6 months post treatment-week acute phase. A novel statistical approach applied to micro-analytic mediator assessment schedule will permit the specification of the most effective CBT component(s) that drive symptom relief.
Discussion: Empirical validation of a low-intensity self-management therapy transdiagnostic in scope has the potential to improve the health of chronic pelvic pain patients refractory to medical therapies, reduce social and economic costs, conserve health care resources, as well as inform evidence-based practice guidelines. Identification of change mechanisms and moderators of treatment effects can provide proactive patient-treatment matching fundamental to goals of personalized medicine.
Trial registration: Clinicaltrials.gov NCT05127616. Registered on 9/19/21.
Keywords: Bladder pain syndrome; Chronic pain; Chronic prostatitis; Interstitial cystitis; Randomized clinical trial; Self-management; Transdiagnostic.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Rodríguez MAB, Afari N, Buchwald DS. National Institute of D, Digestive, Kidney Diseases Working Group on Urological Chronic Pelvic P. Evidence for overlap between urological and nonurological unexplained clinical conditions. J Urol. 2009;182(5):2123–2131. doi: 10.1016/j.juro.2009.07.036. - DOI - PMC - PubMed
-
- Krieger JN, Stephens AJ, Landis JR, et al. Relationship between chronic nonurological associated somatic syndromes and symptom severity in urological chronic pelvic pain syndromes: baseline evaluation of the MAPP study. J Urol. 2015;193(4):1254–1262. doi: 10.1016/j.juro.2014.10.086. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical