Effectiveness of repetitive transcranial magnetic stimulation against poststroke urinary incontinence: a study protocol for a randomized controlled trial
- PMID: 35964135
- PMCID: PMC9375329
- DOI: 10.1186/s13063-022-06535-y
Effectiveness of repetitive transcranial magnetic stimulation against poststroke urinary incontinence: a study protocol for a randomized controlled trial
Abstract
Background and purpose: Poststroke urinary incontinence (PSI) is prevalent in stroke survivors, and high-quality evidence is required to guide clinical practice. Previous studies have demonstrated the curative effect of repetitive transcranial magnetic stimulation (rTMS) for urinary incontinence in individuals with multiple sclerosis (MS), Parkinson's disease (PD), and spinal cord injury (SCI). Here, we describe the protocol for a randomized controlled trial to evaluate the efficacy and safety of low-frequency rTMS on the contralesional primary motor cortex (M1) for the treatment of PSI.
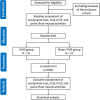
Methods and analysis: In this single-centre randomized controlled trial for poststroke urinary incontinence, a total of 140 eligible patients will be randomly allocated into two groups. The rTMS group (n = 70) will receive low-frequency rTMS at the M1 along with routine medical care, while the control group will receive sham rTMS along with routine medical care. All participants will undergo 20 treatment sessions, five times a week for 4 weeks. The primary outcome measures will be the changes in the urodynamic test at baseline versus 4 weeks after intervention. The secondary outcomes include the International Consultation on Incontinence Questionnaire Urinary Incontinence Short Form (ICIQ-UI SF), Overactive Bladder Symptom Score (OABSS), and pelvic floor muscle function.
Ethics and dissemination: The Institutional Review Board and Hospital Research Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University approved this trial, and the approval number is No. 2020-153. All methods will be carried out in accordance with the principles of the Declaration of Helsinki and relevant ethical guidelines covering informed consent, confidentiality, and data storage. After the study had been thoroughly described to the participants by a physician, all participants will provide written informed consent indicating their willingness to participate. The results will be disseminated to most of the population, including participants, researchers, healthcare providers, and sponsors.
Trial registration: URL: https://www.chictr.org.cn ; Unique identifier: ChiCTR2100042688. Date of Registration: 2021-01-26.
Keywords: Bladder; Clinical trial; Stroke; Urinary incontinence; rTMS.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
A randomized controlled trial of low-frequency repeated transcranial magnetic stimulation in patients with poststroke neurogenic bladder.Sci Rep. 2024 Aug 8;14(1):18404. doi: 10.1038/s41598-024-69345-z. Sci Rep. 2024. PMID: 39117697 Free PMC article. Clinical Trial.
-
Efficacy of biofeedback, repetitive transcranial magnetic stimulation and pelvic floor muscle training for female neurogenic bladder dysfunction after spinal cord injury: a study protocol for a randomised controlled trial.BMJ Open. 2020 Aug 5;10(8):e034582. doi: 10.1136/bmjopen-2019-034582. BMJ Open. 2020. PMID: 32759239 Free PMC article.
-
Comparison of efficacy and safety between electroacupuncture at 'four sacral points' and conventional electroacupuncture for the treatment of urinary incontinence after stroke: study protocol for a randomised controlled trial.BMJ Open. 2018 Nov 5;8(11):e021783. doi: 10.1136/bmjopen-2018-021783. BMJ Open. 2018. PMID: 30397007 Free PMC article.
-
Efficacy of electrical pudendal nerve stimulation versus pelvic floor muscle training in treating postradical prostatectomy urinary incontinence: study protocol for a randomised controlled trial.BMJ Open. 2023 Jan 5;13(1):e062323. doi: 10.1136/bmjopen-2022-062323. BMJ Open. 2023. PMID: 36604129 Free PMC article.
-
Low-Frequency Repetitive Transcranial Magnetic Stimulation in Patients With Poststroke Aphasia: Systematic Review and Meta-Analysis of Its Effect Upon Communication.J Speech Lang Hear Res. 2020 Nov 13;63(11):3801-3815. doi: 10.1044/2020_JSLHR-19-00077. Epub 2020 Oct 20. J Speech Lang Hear Res. 2020. PMID: 33079619
Cited by
-
A randomized controlled trial of low-frequency repeated transcranial magnetic stimulation in patients with poststroke neurogenic bladder.Sci Rep. 2024 Aug 8;14(1):18404. doi: 10.1038/s41598-024-69345-z. Sci Rep. 2024. PMID: 39117697 Free PMC article. Clinical Trial.
References
-
- Tuong NE, Klausner AP, Hampton LJ, et al. Can J Urol. 2016;23(3):8265–8270. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical