How the mechanical microenvironment of stem cell growth affects their differentiation: a review
- PMID: 35964140
- PMCID: PMC9375355
- DOI: 10.1186/s13287-022-03070-0
How the mechanical microenvironment of stem cell growth affects their differentiation: a review
Abstract
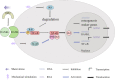
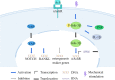
Stem cell differentiation is of great interest in medical research; however, specifically and effectively regulating stem cell differentiation is still a challenge. In addition to chemical factors, physical signals are an important component of the stem cell ecotone. The mechanical microenvironment of stem cells has a huge role in stem cell differentiation. Herein, we describe the knowledge accumulated to date on the mechanical environment in which stem cells exist, which consists of various factors, including the extracellular matrix and topology, substrate stiffness, shear stress, hydrostatic pressure, tension, and microgravity. We then detail the currently known signalling pathways that stem cells use to perceive the mechanical environment, including those involving nuclear factor-kB, the nicotinic acetylcholine receptor, the piezoelectric mechanosensitive ion channel, and hypoxia-inducible factor 1α. Using this information in clinical settings to treat diseases is the goal of this research, and we describe the progress that has been made. In this review, we examined the effects of mechanical factors in the stem cell growth microenvironment on stem cell differentiation, how mechanical signals are transmitted to and function within the cell, and the influence of mechanical factors on the use of stem cells in clinical applications.
Keywords: Extracellular matrix; HIF-1α; Hydrostatic pressure; Microgravity; NF-kB; PIEZO; Shear stress; Stem cell; Tension; nAChR.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
The role of Piezo proteins and cellular mechanosensing in tuning the fate of transplanted stem cells.Cell Tissue Res. 2020 Jul;381(1):1-12. doi: 10.1007/s00441-020-03191-z. Epub 2020 Mar 25. Cell Tissue Res. 2020. PMID: 32215723 Review.
-
[Research status of mechanical stimulation of stem cells differentiation in stem cells microenvironment].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2014 Jan;28(1):100-4. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2014. PMID: 24693789 Review. Chinese.
-
Piezo protein determines stem cell fate by transmitting mechanical signals.Hum Cell. 2023 Mar;36(2):540-553. doi: 10.1007/s13577-022-00853-8. Epub 2022 Dec 29. Hum Cell. 2023. PMID: 36580272 Review.
-
Mechanical Force Directs Proliferation and Differentiation of Stem Cells.Tissue Eng Part B Rev. 2023 Apr;29(2):141-150. doi: 10.1089/ten.TEB.2022.0052. Epub 2022 Oct 10. Tissue Eng Part B Rev. 2023. PMID: 35979892 Review.
-
Regulating osteogenesis and adipogenesis in adipose-derived stem cells by controlling underlying substrate stiffness.J Cell Physiol. 2018 Apr;233(4):3418-3428. doi: 10.1002/jcp.26193. Epub 2017 Nov 10. J Cell Physiol. 2018. PMID: 28926111
Cited by
-
Innovative approaches to boost mesenchymal stem cells efficacy in myocardial infarction therapy.Mater Today Bio. 2025 Jan 9;31:101476. doi: 10.1016/j.mtbio.2025.101476. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 39896290 Free PMC article. Review.
-
Adipo-on-chip: a microphysiological system to culture human mesenchymal stem cells with improved adipogenic differentiation.In Vitro Model. 2024 Oct 9;3(4-6):169-182. doi: 10.1007/s44164-024-00076-1. eCollection 2024 Dec. In Vitro Model. 2024. PMID: 39877645
-
Evaluation of a Novel Mechanical Device for the Production of Microfragmented Adipose Tissue for Veterinary Regenerative Medicine: A Proof-of-Concept.Int J Mol Sci. 2024 Nov 4;25(21):11854. doi: 10.3390/ijms252111854. Int J Mol Sci. 2024. PMID: 39519405 Free PMC article.
-
Physical Stimulation Methods Developed for In Vitro Neuronal Differentiation Studies of PC12 Cells: A Comprehensive Review.Int J Mol Sci. 2024 Jan 7;25(2):772. doi: 10.3390/ijms25020772. Int J Mol Sci. 2024. PMID: 38255846 Free PMC article. Review.
-
Tight Regulation of Mechanotransducer Proteins Distinguishes the Response of Adult Multipotent Mesenchymal Cells on PBCE-Derivative Polymer Films with Different Hydrophilicity and Stiffness.Cells. 2023 Jun 29;12(13):1746. doi: 10.3390/cells12131746. Cells. 2023. PMID: 37443780 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

