Pharmaceutical Potential of Casein-Derived Tripeptide Met-Lys-Pro: Improvement in Cognitive Impairments and Suppression of Inflammation in APP/PS1 Mice
- PMID: 35964178
- PMCID: PMC9535549
- DOI: 10.3233/JAD-220192
Pharmaceutical Potential of Casein-Derived Tripeptide Met-Lys-Pro: Improvement in Cognitive Impairments and Suppression of Inflammation in APP/PS1 Mice
Abstract
Background: Tripeptide Met-Lys-Pro (MKP), a component of casein hydrolysates, has effective angiotensin-converting enzyme (ACE) inhibitory activity. Brain angiotensin II enzyme activates the NADPH oxidase complex via angiotensin II receptor type 1 (AT1) and enhances oxidative stress injury. ACE inhibitors improved cognitive function in Alzheimer's disease (AD) mouse models and previous clinical trials. Thus, although undetermined, MKP may be effective against pathological amyloid-β (Aβ) accumulation-induced cognitive impairment.
Objective: The current study aimed to investigate the potential of MKP as a pharmaceutical against AD by examining MKP's effect on cognitive function and molecular changes in the brain using double transgenic (APP/PS1) mice.
Methods: Experimental procedures were conducted in APP/PS1 mice (n = 38) with a C57BL/6 background. A novel object recognition test was used to evaluate recognition memory. ELISA was used to measure insoluble Aβ40, Aβ42, and TNF-α levels in brain tissue. Immunohistochemical analysis allowed the assessment of glial cell activation in MKP-treated APP/PS1 mice.
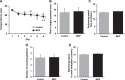
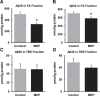
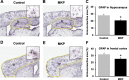
Results: The novel object recognition test revealed that MKP-treated APP/PS1 mice showed significant improvement in recognition memory. ELISA of brain tissue showed that MKP significantly reduced insoluble Aβ40, Aβ42, and TNF-α levels. Immunohistochemical analysis indicated the suppression of the marker for microglia and reactive astrocytes in MKP-treated APP/PS1 mice.
Conclusion: Based on these results, we consider that MKP could ameliorate pathological Aβ accumulation-induced cognitive impairment in APP/PS1 mice. Furthermore, our findings suggest that MKP potentially contributes to preventing cognitive decline in AD.
Keywords: APP/PS1 mice; Alzheimer’s disease; MKP; Met-Lys-Pro; dementia; peptide.
Conflict of interest statement
Authors’ disclosures available online (
Figures
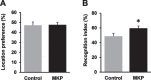


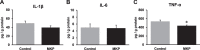
References
-
- Alzheimer’s Disease International (2021)World Alzheimer Report 2021. Journey through the diagnosis of dementia. https://www.alzint.org/u/World-Alzheimer-Report-2021.pdfhttps://www.alzint.org/u/World-Alzheimer-Report-2021.pdf, Last updated September 21, 2021, Accessed on May 23, 2022.
-
- World Health Organization (2021) Dementia. https://www.who.int/news-room/fact.
-
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH (2011) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 280–292. - PMC - PubMed
-
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ (1992) Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production Nature 360, 672–674. - PubMed
-
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics297, 353-356. Erratum in: Science 297, 2209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

