Initial therapeutic results of atezolizumab plus bevacizumab for unresectable advanced hepatocellular carcinoma and the importance of hepatic functional reserve
- PMID: 35964253
- PMCID: PMC9939118
- DOI: 10.1002/cam4.5145
Initial therapeutic results of atezolizumab plus bevacizumab for unresectable advanced hepatocellular carcinoma and the importance of hepatic functional reserve
Abstract
Aim: We analyzed the association between the modified albumin-bilirubin (mALBI) grade and therapeutic efficacy of atezolizumab plus bevacizumab (Atezo+Bev) for the treatment of unresectable hepatocellular carcinoma (u-HCC).
Methods: In this retrospective observational study, we included 71 u-HCC patients treated with Atezo+Bev between September 2020 and September 2021. Patients were grouped corresponding to the mALBI grade at the start of treatment (mALBI 1+2a or mALBI 2b+3) and analyzed for therapeutic effect and the transition rate to secondary treatment.
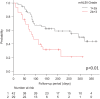
Results: According to the Response Evaluation Criteria in Solid Tumors, the overall response rate was significantly higher for the mALBI 1+2a group, than for the mALBI 2b+3 group, with 26.2% and 3.4%, respectively. The progression-free survival (PFS) was significantly longer in the mALBI 1+2a group (10.5 months) than in the mALBI 2b+3 group (3.0 months). In the multivariate analysis, an mALBI of 1+2a was found to be an independent factor of PFS. The rate of second-line treatment with multi-targeted agents was also significantly higher in the mALBI 1+2a group.
Conclusions: In real-world practice, Atezo+Bev treatment might have higher therapeutic efficacy in u-HCC patients with mALBI 1+2a.
Keywords: atezolizumab; bevacizumab; hepatocellular carcinoma.
© 2022 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Outcomes of Patients with Child-Pugh B and Unresectable Hepatocellular Carcinoma Undergoing First-Line Systemic Treatment with Sorafenib, Lenvatinib, or Atezolizumab Plus Bevacizumab.Oncology. 2024;102(3):239-251. doi: 10.1159/000533859. Epub 2023 Sep 20. Oncology. 2024. PMID: 37729889
-
The potential efficacy of atezolizumab plus bevacizumab treatment for hepatocellular carcinoma patients with macroscopic portal vein tumor thrombus.Surg Today. 2025 Jul;55(7):900-908. doi: 10.1007/s00595-025-03009-x. Epub 2025 Feb 11. Surg Today. 2025. PMID: 39934306
-
Therapeutic efficacy of atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma in patients with Child-Pugh class A or B liver function in real-world clinical practice.Hepatol Res. 2022 Sep;52(9):773-783. doi: 10.1111/hepr.13797. Epub 2022 Jun 11. Hepatol Res. 2022. PMID: 35633504
-
Prognosis and treatment pattern of advanced hepatocellular carcinoma after failure of first-line atezolizumab and bevacizumab treatment.Hepatol Int. 2022 Oct;16(5):1199-1207. doi: 10.1007/s12072-022-10392-x. Epub 2022 Aug 20. Hepatol Int. 2022. PMID: 35986846 Review.
-
Pathological complete response of hepatocellular carcinoma confirmed by conversion hepatectomy following atezolizumab plus bevacizumab therapy: a case report and literature review.Clin J Gastroenterol. 2024 Apr;17(2):292-299. doi: 10.1007/s12328-023-01895-7. Epub 2023 Dec 10. Clin J Gastroenterol. 2024. PMID: 38071671 Free PMC article. Review.
Cited by
-
Durable Stable Disease by Atezolizumab/Bevacizumab Can Provide Long-term Survival of Patients With Hepatocellular Carcinoma Lung Metastases.In Vivo. 2023 Sep-Oct;37(5):2268-2275. doi: 10.21873/invivo.13329. In Vivo. 2023. PMID: 37652506 Free PMC article.
-
Modified albumin-bilirubin predicted survival of unresectable hepatocellular carcinoma patients treated with immunotherapy.World J Gastrointest Oncol. 2023 Oct 15;15(10):1771-1783. doi: 10.4251/wjgo.v15.i10.1771. World J Gastrointest Oncol. 2023. PMID: 37969413 Free PMC article.
-
Initial clinical experience with durvalumab plus tremelimumab in patients with unresectable hepatocellular carcinoma in real‑world practice.Oncol Lett. 2024 Jun 25;28(2):397. doi: 10.3892/ol.2024.14530. eCollection 2024 Aug. Oncol Lett. 2024. PMID: 38979550 Free PMC article.
-
Cost-utility analysis of atezolizumab combined with bevacizumab for unresectable hepatocellular carcinoma in Thailand.PLoS One. 2024 Mar 21;19(3):e0300327. doi: 10.1371/journal.pone.0300327. eCollection 2024. PLoS One. 2024. PMID: 38512900 Free PMC article.
-
Heterogeneity in adverse events related to atezolizumab-bevacizumab for hepatocellular carcinoma reported in real-world studies.JHEP Rep. 2024 Aug 22;6(11):101190. doi: 10.1016/j.jhepr.2024.101190. eCollection 2024 Nov. JHEP Rep. 2024. PMID: 39524204 Free PMC article.
References
-
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301‐1314. - PubMed
-
- Nault JC, Galle PR, Marquardt JU. The role of molecular enrichment on future therapies in hepatocellular carcinoma. J Hepatol. 2018;69(1):237‐247. - PubMed
-
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894‐1905. - PubMed
-
- Llovet JM, Villanueva A, Marrero JA, et al. AASLD panel of experts on trial design in HCC. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference. Hepatology. 2021;73(Suppl 1):158‐191. - PubMed
-
- Vogel A, Martinelli E, ESMO Guidelines Committee , Electronic address: clinicalguidelines@ESMO.Org, ESMO Guidelines Committee . Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO clinical practice Guidelines. Ann Oncol. 2021;32(6):801‐805. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

