Increased NOS coupling by the metabolite tetrahydrobiopterin (BH4) reduces preeclampsia/IUGR consequences
- PMID: 35964341
- PMCID: PMC9389306
- DOI: 10.1016/j.redox.2022.102406
Increased NOS coupling by the metabolite tetrahydrobiopterin (BH4) reduces preeclampsia/IUGR consequences
Abstract
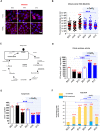
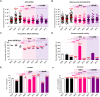
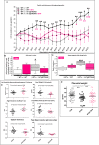
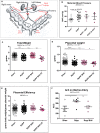
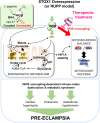
Preeclampsia (PE) is a high-prevalence pregnancy disease characterized by placental insufficiency, gestational hypertension, and proteinuria. Overexpression of the A isoform of the STOX1 transcription factor (STOX1A) recapitulates PE in mice, and STOX1A overexpressing trophoblasts recapitulate PE patients hallmarks in terms of gene expression and pathophysiology. STOX1 overexpression induces nitroso-redox imbalance and mitochondrial hyper-activation. Here, by a thorough analysis on cell models, we show that STOX1 overexpression in trophoblasts alters inducible nitric oxide synthase (iNOS), nitric oxide (NO) content, the nitroso-redox balance, the antioxidant defense, and mitochondrial function. This is accompanied by specific alterations of the Krebs cycle leading to reduced l-malate content. By increasing NOS coupling using the metabolite tetrahydrobiopterin (BH4) we restore this multi-step pathway in vitro. Moving in vivo on two different rodent models (STOX1 mice and RUPP rats, alike early onset and late onset preeclampsia, respectively), we show by transcriptomics that BH4 directly reverts STOX1-deregulated gene expression including glutathione metabolism, oxidative phosphorylation, cholesterol metabolism, inflammation, lipoprotein metabolism and platelet activation, successfully treating placental hypotrophy, gestational hypertension, proteinuria and heart hypertrophy. In the RUPP rats we show that the major fetal issue of preeclampsia, Intra Uterine Growth Restriction (IUGR), is efficiently corrected. Our work posits on solid bases BH4 as a novel potential therapy for preeclampsia.
Keywords: Malate; Mitochondria; Nitroso-redox balance; Preeclampsia; STOX1; Tetrahydrobiopterin; iNOS.
Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

