ER and Golgi trafficking in axons, dendrites, and glial processes
- PMID: 35964523
- PMCID: PMC9590103
- DOI: 10.1016/j.ceb.2022.102119
ER and Golgi trafficking in axons, dendrites, and glial processes
Abstract
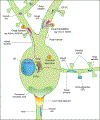
Both neurons and glia in mammalian brains are highly ramified. Neurons form complex neural networks using axons and dendrites. Axons are long with few branches and form pre-synaptic boutons that connect to target neurons and effector tissues. Dendrites are shorter, highly branched, and form post-synaptic boutons. Astrocyte processes contact synapses and blood vessels in order to regulate neuronal activity and blood flow, respectively. Oligodendrocyte processes extend toward axons to make myelin sheaths. Microglia processes dynamically survey their environments. Here, we describe the local secretory system (ER and Golgi) in neuronal and glial processes. We focus on Golgi outpost functions in acentrosomal microtubule nucleation, cargo trafficking, and protein glycosylation. Thus, satellite ER and Golgi are critical for local structure and function in neurons and glia.
Published by Elsevier Ltd.
Conflict of interest statement
Conflict of interest statement Nothing declared.
Figures

References
-
- Song AH, Wang D, Chen G, Li Y, Luo J, Duan S, Poo MM: A selective filter for cytoplasmic transport at the axon initial segment. Cell 2009, 136:1148–60. - PubMed
-
-
Bourke AM, Schwartz SL, Bowen AB, Kleinjan MS, Winborn CS, Kareemo DJ, Gutnick A, Schwarz TL, Kennedy MJ: zapERtrap: A light-regulated ER release system reveals unexpected neuronal trafficking pathways. J Cell Biol 2021, 220:e202103186.
* This study developed a live-cell imaging tool that uses a focused pulse of light to trigger the release of proteins from either the cell body or dendritic ER. Interestingly, the authors found that NL1 can be trafficked from the cell body ER to the plasma membrane of the AIS.
-

