Mepolizumab for urban children with exacerbation-prone eosinophilic asthma in the USA (MUPPITS-2): a randomised, double-blind, placebo-controlled, parallel-group trial
- PMID: 35964610
- PMCID: PMC9623810
- DOI: 10.1016/S0140-6736(22)01198-9
Mepolizumab for urban children with exacerbation-prone eosinophilic asthma in the USA (MUPPITS-2): a randomised, double-blind, placebo-controlled, parallel-group trial
Abstract
Background: Black and Hispanic children living in urban environments in the USA have an excess burden of morbidity and mortality from asthma. Therapies directed at the eosinophilic phenotype reduce asthma exacerbations in adults, but few data are available in children and diverse populations. Furthermore, the molecular mechanisms that underlie exacerbations either being prevented by, or persisting despite, immune-based therapies are not well understood. We aimed to determine whether mepolizumab, added to guidelines-based care, reduced the number of asthma exacerbations during a 52-week period compared with guidelines-based care alone.
Methods: This is a randomised, double-blind, placebo-controlled, parallel-group trial done at nine urban medical centres in the USA. Children and adolescents aged 6-17 years, who lived in socioeconomically disadvantaged neighbourhoods and had exacerbation-prone asthma (defined as ≥two exacerbations in the previous year) and blood eosinophils of at least 150 cells per μL were randomly assigned 1:1 to mepolizumab (6-11 years: 40 mg; 12-17 years: 100 mg) or placebo injections once every 4 weeks, plus guideline-based care, for 52 weeks. Randomisation was done using a validated automated system. Participants, investigators, and the research staff who collected outcome measures remained masked to group assignments. The primary outcome was the number of asthma exacerbations that were treated with systemic corticosteroids during 52 weeks in the intention-to-treat population. The mechanisms of treatment response were assessed by study investigators using nasal transcriptomic modular analysis. Safety was assessed in the intention-to-treat population. This trial is registered with ClinicalTrials.gov, NCT03292588.
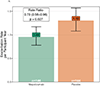
Findings: Between Nov 1, 2017, and Mar 12, 2020, we recruited 585 children and adolescents. We screened 390 individuals, of whom 335 met the inclusion criteria and were enrolled. 290 met the randomisation criteria, were randomly assigned to mepolizumab (n=146) or placebo (n=144), and were included in the intention-to-treat analysis. 248 completed the study. The mean number of asthma exacerbations within the 52-week study period was 0·96 (95% CI 0·78-1·17) with mepolizumab and 1·30 (1·08-1·57) with placebo (rate ratio 0·73; 0·56-0·96; p=0·027). Treatment-emergent adverse events occurred in 42 (29%) of 146 participants in the mepolizumab group versus 16 (11%) of 144 participants in the placebo group. No deaths were attributed to mepolizumab.
Interpretation: Phenotype-directed therapy with mepolizumab in urban children with exacerbation-prone eosinophilic asthma reduced the number of exacerbations.
Funding: US National Institute of Allergy and Infectious Diseases and GlaxoSmithKline.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests All authors, with the exceptions of PMB, LG, and PJG, report grants from the US National Institutes of Health (NIH)–NIAID during the study. CMV, AC, SW, LG, PJG, and PMB have nothing to disclose outside of the submitted work. LBB reports personal fees from GlaxoSmithKline, Genentech–Novartis, Teva, Boehringer Ingelheim, AstraZeneca, WebMD–Medscape, Merck, DBV Technologies, Circassia, Sanofi–Regeneron, and Vectura, all outside the submitted work. GTO reports personal fees from AstraZeneca and grant funding from Janssen Pharmaceuticals, outside the submitted work. CMD reports personal fees from Horizon Therapeutics and Enzyvant Therapeutics, outside the submitted work. MCA reports personal fees from Sanofi–Regeneron, outside the submitted work. WWB reports personal fees from Boston Scientific, Novartis, GlaxoSmithKline, Genentech, Sanofi–Genzyme, AstraZeneca, Teva, Regeneron, and Elsevier, outside the submitted work. MAG reports an honorarium for and support for travel to the 2017 American Academy of Allergy, Asthma & Immunology meeting during this study and monetary compensation from the American Academy of Pediatrics for her work teaching the biannual Pediatrics board review course: PREP The Course. GKKH reports grants from Adare during the study. DJJ reports personal fees from Novartis, Pfizer, Regeneron, AstraZeneca, Sanofi, and Vifor Pharma; grants and personal fees from GlaxoSmithKline; and grants from NIH–US National Heart, Lung, and Blood Institute (NHLBI), all outside the submitted work. MK reports personal fees from Regeneron, outside the submitted work. RSG reports government employment from the Center for Biologics Evaluation and Research and personal fees from Consulting Massachusetts Medical Society, outside the submitted work. AHL reports personal fees from Phadia ThermoFisher as consulting honoraria; grants and non-financial support from ResMed–Propeller Health; non-financial support from Revenio; grants and personal fees from Avillion; and personal fees from Labcorp, all outside the submitted work. SJT reports grants from NIH–Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH–NHLBI, and EJF Philanthropies and personal fees from Uptodate, all outside the submitted work. JAP reports provision of study drug for another asthma research study from GlaxoSmithKline and Boehringer Ingelheim and provision of study drug for another asthma research study and for food allergy research studies from Genentech–Novartis, all outside the submitted work. All other authors declare no competing interests.
Figures



Comment in
-
Biologic therapies for asthma in underserved populations.Lancet. 2022 Aug 13;400(10351):471-473. doi: 10.1016/S0140-6736(22)01383-6. Lancet. 2022. PMID: 35964595 Free PMC article. No abstract available.
References
-
- O'Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179(1):19–24. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

