Early drug development in solid tumours: analysis of National Cancer Institute-sponsored phase 1 trials
- PMID: 35964611
- PMCID: PMC9477645
- DOI: 10.1016/S0140-6736(22)01390-3
Early drug development in solid tumours: analysis of National Cancer Institute-sponsored phase 1 trials
Abstract
Background: The low expectation of clinical benefit from phase 1 cancer therapeutics trials might negatively affect patient and physician participation, study reimbursement, and slow the progress of oncology research. Advances in cancer drug development, meanwhile, might have favourably improved treatment responses; however, little comprehensive data exist describing the response and toxicity associated with phase 1 trials across solid tumours. The aim of the study is to evaluate the trend of toxicity and response in phase 1 trials for solid tumours over time.
Methods: We analysed patient-level data from the Cancer Therapy Evaluation Program of the National Cancer Institute-sponsored investigator-initiated phase 1 trials for solid tumours, from Jan 1, 2000, to May 31, 2019. We assessed risks of treatment-related death (grade 5 toxicity ratings possibly, probably, or definitely attributable to treatment), all on-treatment deaths (deaths during protocol treatment regardless of attribution), grade 3-4 toxicity, and proportion of overall response (complete response and partial response) and complete response rate in the study periods of 2000-05, 2006-12, and 2013-2019, and evaluated their trends over time. We also analysed cancer type-specific and investigational agent-specific response, and analysed the trend of response in each cancer type over time. Univariate associations of overall response rates with patients' baseline characteristics (age, sex, performance status, BMI, albumin concentration, and haemoglobin concentration), enrolment period, investigational agents, and trial design were assessed using risk ratio based on the modified Poisson regression model.
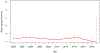
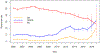
Findings: We analysed 465 protocols that enrolled 13 847 patients using 261 agents. 144 (31%) trials used a monotherapy and 321 (69%) used combination therapies. The overall treatment-related death rate was 0·7% (95% CI 0·5-0·8) across all periods. Risks of treatment-related deaths did not change over time (p=0·52). All on-treatment death risk during the study period was 8·0% (95% CI 7·6-8·5). The most common grade 3-4 adverse events were haematological; grade 3-4 neutropenia occurred in 2336 (16·9%) of 13 847 patients, lymphopenia in 1230 (8·9%), anaemia in 894 (6·5%), and thrombocytopenia in 979 (7·1%). The overall response rate for all trials during the study period was 12·2% (95% CI 11·5-12·8; 1133 of 9325 patients) and complete response rate was 2·7% (2·4-3·0; 249 of 9325). Overall response increased from 9·6% (95% CI 8·7-10·6) in 2000-05 to 18·0% (15·7-20·5) in 2013-19, and complete response rates from 2·5% (2·0-3·0) to 4·3% (3·2-5·7). Overall response rates for combination therapy were substantially higher than for monotherapy (15·8% [15·0-16·8] vs 3·5% [2·8-4·2]). The overall response by class of agents differed across diseases. Anti-angiogenesis agents were associated with higher overall response rate for bladder, colon, kidney and ovarian cancer. DNA repair inhibitors were associated with higher overall response rate in ovarian and pancreatic cancer. The rates of overall response over time differed markedly by disease; there were notable improvements in bladder, breast, and kidney cancer and melanoma, but no change in the low response of pancreatic and colon cancer.
Interpretation: During the past 20 years, the response rate in phase 1 trials nearly doubled without an increase in the treatment-related death rate. However, there is significant heterogeneity in overall response by various factors such as cancer type, investigational agent, and trial design. Therefore, informed decision making is crucial for patients before participating in phase 1 trials. This study provides updated encouraging outcomes of modern phase 1 trials in solid tumours.
Funding: National Cancer Institute.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests RL has served as a consultant for Monte Rosa Therapeutics. CRF has served as a consultant for AbbVie, AstraZeneca, Bayer, BeiGene, Bristol Meyers Sqibb/Celgene, Denovo Biopharma, Genentech/Roche Pharma, Genmab, Gilead Sciences, Karyopharm Therapeutics, Morphosys, Pharmacyclics/Janssen, Seagen, and Spectrum Pharmaceuticals; and has received research funding paid to the institution from 4D, AbbVie, Acerta Pharma, Adaptimmune, Allogene Therapeutics, Amgen, Bayer, Celgene, Cellectis, EMD, Gilead Sciences, Genentech/Roche, Guardant, Iovance Biotherapeutics, Janssen, Kite Pharma, MorphoSys, Nektar Therapeutics, Novartis, Pfizer, Pharmacyclics, Sanofi, Takeda, TG Therapeutics, Xencor, Ziopharm, Burroughs Wellcome Fund, Eastern Cooperative Oncology Group, National Cancer Institute, V Foundation for Cancer Research, and the Cancer Prevention and Research Institute of Texas where he is a CPRIT Scholar in Cancer Research. LJN has received research support from BMS/Celgene, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, IGM Biosciences, Janssen, Novartis, Takeda, and TG Therapeutics; and has served as consultant for ADC Therapeutics, Bayer, BMS/Celgene, Epizyme, Genentech, Gilead/Kite, Janssen, Morphosys, Novartis, Takeda, and TG Therapeutics. All other authors declare no competing interests.
Figures
Comment in
-
Participation in phase 1 trials for patients with cancer.Lancet. 2022 Aug 13;400(10351):473-475. doi: 10.1016/S0140-6736(22)01533-1. Lancet. 2022. PMID: 35964596 No abstract available.
Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Characteristics and outcomes of breast cancer patients enrolled in the National Cancer Institute Cancer Therapy Evaluation Program sponsored phase I clinical trials.Breast Cancer Res Treat. 2018 Feb;168(1):35-41. doi: 10.1007/s10549-017-4563-3. Epub 2017 Nov 8. Breast Cancer Res Treat. 2018. PMID: 29119354 Free PMC article.
-
Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, phase 1 trial.Lancet Oncol. 2017 Jun;18(6):770-778. doi: 10.1016/S1470-2045(17)30314-5. Epub 2017 May 8. Lancet Oncol. 2017. PMID: 28495639 Free PMC article. Clinical Trial.
-
Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial.Lancet Oncol. 2017 May;18(5):587-598. doi: 10.1016/S1470-2045(17)30239-5. Epub 2017 Mar 31. Lancet Oncol. 2017. PMID: 28373007 Free PMC article. Clinical Trial.
-
Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.Drugs. 2003;63(8):803-43. doi: 10.2165/00003495-200363080-00005. Drugs. 2003. PMID: 12662126 Review.
Cited by
-
Changing trends in phase 1 oncology clinical trials.Contemp Clin Trials Commun. 2023 Dec 18;37:101239. doi: 10.1016/j.conctc.2023.101239. eCollection 2024 Feb. Contemp Clin Trials Commun. 2023. PMID: 38204884 Free PMC article.
-
Project Optimus, an FDA initiative: Considerations for cancer drug development internationally, from an academic perspective.Front Oncol. 2023 Mar 3;13:1144056. doi: 10.3389/fonc.2023.1144056. eCollection 2023. Front Oncol. 2023. PMID: 36937434 Free PMC article.
-
Travel Time and Distance and Participation in Precision Oncology Trials at the National Cancer Center Hospital.JAMA Netw Open. 2023 Sep 5;6(9):e2333188. doi: 10.1001/jamanetworkopen.2023.33188. JAMA Netw Open. 2023. PMID: 37713200 Free PMC article.
-
Relevance, Risks, and Benefits of Early-Phases Clinical Trials Participations for Patients With Hematological Malignancies From 2008 to 2023.Eur J Haematol. 2025 Jan;114(1):89-97. doi: 10.1111/ejh.14307. Epub 2024 Sep 21. Eur J Haematol. 2025. PMID: 39305190 Free PMC article.
-
COCA: a randomized Bayesian design integrating dose optimization and component contribution assessment for combination therapies.Biometrics. 2025 Apr 2;81(2):ujaf077. doi: 10.1093/biomtc/ujaf077. Biometrics. 2025. PMID: 40552493
References
-
- Horstmann E, McCabe MS, Grochow L, et al. Risks and benefits of phase 1 oncology trials, 1991 through 2002. N Engl J Med 2005; 352(9): 895–904. - PubMed
-
- Roberts TG Jr., Goulart BH, Squitieri L, et al. Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials. JAMA 2004; 292(17): 2130–40. - PubMed
-
- Von Hoff DD, Turner J. Response rates, duration of response, and dose response effects in phase I studies of antineoplastics. Invest New Drugs 1991; 9(1): 115–22. - PubMed
-
- Decoster G, Stein G, Holdener EE. Responses and toxic deaths in phase I clinical trials. Ann Oncol 1990; 1(3): 175–81. - PubMed
-
- Estey E, Hoth D, Simon R, Marsoni S, Leyland-Jones B, Wittes R. Therapeutic response in phase I trials of antineoplastic agents. Cancer Treat Rep 1986; 70(9): 1105–15. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous