Safety of COVID-19 vaccines in pregnancy: a Canadian National Vaccine Safety (CANVAS) network cohort study
- PMID: 35964614
- PMCID: PMC9371587
- DOI: 10.1016/S1473-3099(22)00426-1
Safety of COVID-19 vaccines in pregnancy: a Canadian National Vaccine Safety (CANVAS) network cohort study
Abstract
Background: Pregnant individuals have been receiving COVID-19 vaccines following pre-authorisation clinical trials in non-pregnant people. This study aimed to determine the frequency and nature of significant health events among pregnant females after COVID-19 vaccination, compared with unvaccinated pregnant controls and vaccinated non-pregnant individuals.
Methods: We did an observational cohort study, set in seven Canadian provinces and territories including Ontario, Quebec, British Columbia, Alberta, Nova Scotia, Yukon, and Prince Edward Island. Eligibility criteria for vaccinated individuals were a first dose of a COVID-19 vaccine within the previous 7 days; an active email address and telephone number; ability to communicate in English or French; and residence in the aforementioned provinces or territories. Study participants were pregnant and non-pregnant females aged 15-49 years. Individuals were able to participate as controls if they were unvaccinated and fulfilled the other criteria. Data were collected primarily by self-reported survey after both vaccine doses, with telephone follow-up for those reporting any medically attended event. Participants reported significant health events (new or worsening of a health event sufficient to cause work or school absenteeism, medical consultation, or prevent daily activities) occurring within 7 days of vaccination or within the past 7 days for unvaccinated individuals. We employed multivariable logistic regression to examine significant health events associated with mRNA vaccines, adjusting for age group, previous SARS-CoV-2 infection, and trimester, as appropriate.
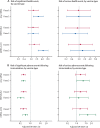
Findings: As of Nov 4, 2021, 191 360 women aged 15-49 years with known pregnancy status had completed the first vaccine dose survey and 94 937 had completed the second dose survey. 180 388 received one dose and 94 262 received a second dose of an mRNA vaccine, with 5597 pregnant participants receiving dose one and 3108 receiving dose two, and 174 765 non-pregnant participants receiving dose one and 91 131 receiving dose two. Of 6179 included unvaccinated control participants, 339 were pregnant and 5840 were not pregnant. Overall, 226 (4·0%) of 5597 vaccinated pregnant females reported a significant health event after dose one of an mRNA vaccine, and 227 (7·3%) of 3108 after dose two, compared with 11 (3·2%) of 339 pregnant unvaccinated females. Pregnant vaccinated females had an increased odds of a significant health event within 7 days of the vaccine after dose two of mRNA-1273 (adjusted odds ratio [aOR] 4·4 [95% CI 2·4-8·3]) compared with pregnant unvaccinated controls within the past 7 days, but not after dose one of mRNA-1273 or any dose of BNT162b2. Pregnant vaccinated females had decreased odds of a significant health event compared with non-pregnant vaccinated females after both dose one (aOR 0·63 [95% CI 0·55-0·72]) and dose two (aOR 0·62 [0·54-0·71]) of any mRNA vaccination. There were no significant differences in any analyses when restricted to events which led to medical attention.
Interpretation: COVID-19 mRNA vaccines have a good safety profile in pregnancy. These data can be used to appropriately inform pregnant people regarding reactogenicity of COVID-19 vaccines during pregnancy, and should be considered alongside effectiveness and immunogenicity data to make appropriate recommendations about best use of COVID-19 vaccines in pregnancy.
Funding: Canadian Institutes of Health Research, Public Health Agency of Canada.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests MS has been an investigator on projects funded by GlaxoSmithKline, Merck, Moderna, Pfizer, Sanofi-Pasteur, Seqirus, Symvivo, and VBI Vaccines. All funds have been paid to his institute, and he has not received any personal payments. OGV has been an investigator, coinvestigator, or expert panelist on projects funded by GlaxoSmithKline, Merck, Pfizer, and Seqirus, outside of the submitted work. JDK has been an investigator on projects funded by GlaxoSmithKline, Merck, Moderna, and Pfizer. All funds have been paid to his institute, and he has not received any personal payments. KAT has been an investigator on projects funded by GlaxoSmithKline. All funds have been paid to her institute, and she has not received any personal payments. JEI has been an investigator on projects funded by GlaxoSmithKline, and Sanofi-Pasteur. All funds have been paid to her institute, and she has not received any personal payments. AJM has been an investigator on projects funded by GlaxoSmithKline, Merck, Pfizer, Sanofi-Pasteur, and Seqirus, with funds paid to her institution, and has received honoraria for participation in advisory boards from Astra-Zeneca, GlaxoSmithKline, Medicago, Merck, Moderna, Pfizer, Sanofi-Pasteur, Seqirus, and for presentations from Astra-Zeneca, and Moderna. GDS has been an investigator on a project funded by Pfizer. All funds have been paid to his institute, and he has not received any personal payments. All other authors declare no competing interests.
Figures

Comment in
-
Safety of mRNA COVID-19 vaccines during pregnancy.Lancet Infect Dis. 2022 Nov;22(11):1514-1515. doi: 10.1016/S1473-3099(22)00443-1. Epub 2022 Aug 11. Lancet Infect Dis. 2022. PMID: 35964615 Free PMC article. No abstract available.
Similar articles
-
mRNA COVID-19 vaccine safety among older adults from the Canadian National Vaccine Safety Network.Vaccine. 2024 Jul 11;42(18):3819-3829. doi: 10.1016/j.vaccine.2024.04.096. Epub 2024 May 6. Vaccine. 2024. PMID: 38714447
-
Association Between BNT162b2 Vaccination and Incidence of SARS-CoV-2 Infection in Pregnant Women.JAMA. 2021 Aug 24;326(8):728-735. doi: 10.1001/jama.2021.11035. JAMA. 2021. PMID: 34251417 Free PMC article.
-
Immunogenicity and safety of a booster dose of a self-amplifying RNA COVID-19 vaccine (ARCT-154) versus BNT162b2 mRNA COVID-19 vaccine: a double-blind, multicentre, randomised, controlled, phase 3, non-inferiority trial.Lancet Infect Dis. 2024 Apr;24(4):351-360. doi: 10.1016/S1473-3099(23)00650-3. Epub 2023 Dec 20. Lancet Infect Dis. 2024. PMID: 38141632 Clinical Trial.
-
Safety and effectiveness of vaccines against COVID-19 in children aged 5-11 years: a systematic review and meta-analysis.Lancet Child Adolesc Health. 2023 Jun;7(6):379-391. doi: 10.1016/S2352-4642(23)00078-0. Epub 2023 Apr 18. Lancet Child Adolesc Health. 2023. PMID: 37084750 Free PMC article.
-
Efficacy and Clinical Outcomes of mRNA COVID-19 Vaccine in Pregnancy: A Systematic Review and Meta-Analysis.Intervirology. 2024;67(1):40-54. doi: 10.1159/000538135. Epub 2024 Mar 2. Intervirology. 2024. PMID: 38432215 Free PMC article.
Cited by
-
Pregnancy and COVID-19: past, present and future.Obstet Gynecol Sci. 2023 May;66(3):149-160. doi: 10.5468/ogs.23001. Epub 2023 Mar 20. Obstet Gynecol Sci. 2023. PMID: 36938588 Free PMC article.
-
Effectiveness and safety of COVID-19 vaccines on maternal and perinatal outcomes: a systematic review and meta-analysis.BMJ Glob Health. 2024 Apr 4;9(4):e014247. doi: 10.1136/bmjgh-2023-014247. BMJ Glob Health. 2024. PMID: 38580375 Free PMC article.
-
Safety of COVID-19 Vaccines Among Pregnant Women in India: A Systematic Review and Meta-Analysis.Cureus. 2025 Jun 16;17(6):e86115. doi: 10.7759/cureus.86115. eCollection 2025 Jun. Cureus. 2025. PMID: 40671971 Free PMC article. Review.
-
Efficacy, safety, and public attitude toward COVID-19 vaccines: A systematic review.Ann Afr Med. 2023 Oct-Dec;22(4):405-414. doi: 10.4103/aam.aam_13_23. Ann Afr Med. 2023. PMID: 38358138 Free PMC article.
-
Perinatal Outcomes at Birth in Women Infected and Non-Infected with SARS-CoV-2: A Retrospective Study.Healthcare (Basel). 2023 Oct 27;11(21):2833. doi: 10.3390/healthcare11212833. Healthcare (Basel). 2023. PMID: 37957979 Free PMC article.
References
-
- National Advisory Committee on Immunization Recommendations on the use of COVID-19 vaccines. 2020. https://www.canada.ca/en/public-health/services/immunization/national-ad...
-
- Poliquin V, Castillo E, Boucoiran I, et al. Society of Obstetricians and Gynecologists of Canada; Ottawa, ON, Canada: 2020. SOGC statement on COVID-19 vaccination in pregnancy.
-
- The American College of Obstetricians and Gynecologists COVID-19 vaccination considerations for obstetric-gynecologic care. 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articl...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

