False-positive Xpert® Xpress SARS-CoV-2 assay in an emergency room and trauma center: A retrospective chart review study
- PMID: 35964955
- PMCID: PMC9749665
- DOI: 10.15537/smj.2022.43.8.20220317
False-positive Xpert® Xpress SARS-CoV-2 assay in an emergency room and trauma center: A retrospective chart review study
Abstract
Objectives: To review reports false-positive Xpert results in an emergency room and trauma center.
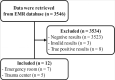
Methods: Patients' data with false-positive Xpert results from November 2020 to February 2022 at Pusan National University Hospital, Busan, Republic of Korea, were extracted from the electronic medical records.
Results: The positive predictive value of Xpert was 40%. Of the 12 patients with false-positive results, 5 (41.7%) were re-positives (such as, patients recovered from coronavirus disease-19 [COVID-19]), and 4 (33.3%) had head or facial trauma. Two out of 4 head or facial trauma cases had documented sample contamination with blood.
Conclusion: We found a high incidence of false-positive Xpert results among patients who recovered from COVID-19 and those with head or facial injury. Careful history taking for COVID-19 and physical examination of the sample collection site is essential before Xpert analysis.
Keywords: COVID-19; COVID-19 nucleic acid testing; SARS-CoV-2; false positive reactions; polymerase chain reaction.
Copyright: © Saudi Medical Journal.
Figures
Similar articles
-
Diagnostic accuracy of the Cepheid Xpert Xpress and the Abbott ID NOW assay for rapid detection of SARS-CoV-2: A systematic review and meta-analysis.J Med Virol. 2021 Jul;93(7):4523-4531. doi: 10.1002/jmv.26994. Epub 2021 May 3. J Med Virol. 2021. PMID: 33913533 Free PMC article.
-
Comparison of Xpert Xpress SARS-CoV-2 Assay Compared with Standard M nCoV Real-Time PCR: Prospective Study.Clin Lab. 2024 Feb 1;70(2). doi: 10.7754/Clin.Lab.2023.230802. Clin Lab. 2024. PMID: 38345984
-
European multicenter evaluation of Xpert® Xpress SARS-CoV-2/Flu/RSV test.J Med Virol. 2021 Oct;93(10):5798-5804. doi: 10.1002/jmv.27111. Epub 2021 Jun 6. J Med Virol. 2021. PMID: 34050951 Free PMC article.
-
Multi-center evaluation of cepheid xpert® xpress SARS-CoV-2 point-of-care test during the SARS-CoV-2 pandemic.J Clin Virol. 2020 Jul;128:104426. doi: 10.1016/j.jcv.2020.104426. Epub 2020 May 11. J Clin Virol. 2020. PMID: 32417674 Free PMC article.
-
Potential false-positive reasons for SARS-CoV-2 antibody testing and its solution.J Med Virol. 2021 Jul;93(7):4242-4246. doi: 10.1002/jmv.26937. Epub 2021 Mar 25. J Med Virol. 2021. PMID: 33710634 Free PMC article. Review.
References
-
- Das R, Joshi S, Pednekar S, Karyakarte R. Comparison of Xpert Xpress SARS-CoV-2 assay and RT-PCR test in diagnosis of COVID-19. IOSR J Dent Med Sci 2021; 20: 12–17.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous