Measurement of ATP utilization in RNA unwinding and RNA chaperone activities by DEAD-box helicase proteins
- PMID: 35965018
- PMCID: PMC10040262
- DOI: 10.1016/bs.mie.2022.04.004
Measurement of ATP utilization in RNA unwinding and RNA chaperone activities by DEAD-box helicase proteins
Abstract
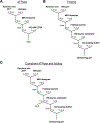
RNA helicase proteins perform coupled reactions in which cycles of ATP binding and hydrolysis are used to drive local unwinding of double-stranded RNA (dsRNA). For some helicases in the ubiquitous DEAD-box family, these local unwinding events are integral to folding transitions in structured RNAs, and thus these helicases function as RNA chaperones. An important measure of the efficiency of the helicase-catalyzed reaction is the ATP utilization value, which represents the average number of ATP molecules hydrolyzed during RNA unwinding or a chaperone-assisted RNA structural rearrangement. Here we outline procedures that can be used to measure the ATP utilization value in RNA unwinding or folding transitions. As an example of an RNA folding transition, we focus on the refolding of the Tetrahymena thermophila group I intron ribozyme from a long-lived misfolded structure to its native structure, and we outline strategies for adapting this assay to other RNA folding transitions. For a simple dsRNA unwinding event, the ATP utilization value provides a measure of the coupling between the ATPase and RNA unwinding activities, and for a complex RNA structural transition it can give insight into the scope of the rearrangement and the efficiency with which the helicase uses the energy from ATPase cycles to promote the rearrangement.
Keywords: ATPase kinetics; CYT-19; DEAD-box protein; Double-stranded RNA; RNA chaperone; RNA helicase; RNA misfolding.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures




References
-
- Atkinson A, & Jack GW (1973). Precipitation of nucleic acids with polyethyleneimine and the chromatography of nucleic acids and proteins on immobilised polyethyleneimine. Biochimica et Biophysica Acta (BBA) - Nucleic Acids and Protein Synthesis, 308(1), 41–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

