Intercontinental Diversity of Caballeronia Gut Symbionts in the Conifer Pest Bug Leptoglossus occidentalis
- PMID: 35965097
- PMCID: PMC9530724
- DOI: 10.1264/jsme2.ME22042
Intercontinental Diversity of Caballeronia Gut Symbionts in the Conifer Pest Bug Leptoglossus occidentalis
Abstract
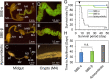
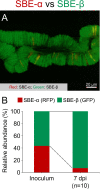
Many stinkbugs in the superfamily Coreoidea (Hemiptera: Heteroptera) develop crypts in the posterior midgut, harboring Caballeronia (Burkholderia) symbionts. These symbionts form a monophyletic group in Burkholderia sensu lato, called the "stinkbug-associated beneficial and environmental (SBE)" group, recently reclassified as the new genus Caballeronia. SBE symbionts are separated into the subclades SBE-α and SBE-β. Previous studies suggested a regional effect on the symbiont infection pattern; Japanese and American bug species are more likely to be associated with SBE-α, while European bug species are almost exclusively associated with SBE-β. However, since only a few insect species have been investigated, it remains unclear whether region-specific infection is general. We herein investigated Caballeronia gut symbionts in diverse Japanese, European, and North American populations of a cosmopolitan species, the Western conifer seed bug Leptoglossus occidentalis (Coreoidea: Coreidae). A mole-cular phylogenetic ana-lysis of the 16S rRNA gene demonstrated that SBE-β was the most dominant in all populations. Notably, SBE-α was rarely detected in any region, while a third clade, the "Coreoidea clade" occupied one fourth of the tested populations. Although aposymbiotic bugs showed high mortality, SBE-α- and SBE-β-inoculated insects both showed high survival rates; however, a competition assay demonstrated that SBE-β outcompeted SBE-α in the midgut crypts of L. occidentalis. These results strongly suggest that symbiont specificity in the Leptoglossus-Caballeronia symbiotic association is influenced by the host rather than geography, while the geographic distribution of symbionts may be more important in other bugs.
Keywords: Caballeronia; intercontinental diversity; obligate gut symbiosis; stinkbug.
Figures



References
-
- Ahn, S.J., Son, D., Choo, H.Y., and Park, C.G. (2013) The first record on Leptoglossus occidentalis (Hemiptera: Coreidae) in Korea, a potential pest of the pinaceous tree species. J Asia-Pac Entomol 16: 281–284.
-
- Ben Jamaa, M., Mejri, M., Naves, P., and Sousa, E. (2013) Detection of Leptoglossus occidentalis Heidemann, 1910 (Heteroptera: Coreidae) in Tunisia. Afr Entomol 21: 165–167.

