COVID-19 vaccine uptake among Arkansas public K-12 school teachers and staff
- PMID: 35965240
- PMCID: PMC9343748
- DOI: 10.1016/j.vaccine.2022.07.045
COVID-19 vaccine uptake among Arkansas public K-12 school teachers and staff
Abstract
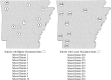
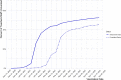
In December 2020, the first coronavirus disease 2019 (COVID-19) vaccines received emergency use authorization from the Food and Drug Administration (FDA). To strategically allocate the limited availability of COVID-19 vaccines, the Advisory Committee on Immunization Practices (ACIP) developed a phased approach for eligibility that prioritized certain population groups that were more vulnerable to infection and severe outcomes. Public K-12 teachers and staff were included in Phase 1b. The Arkansas Department of Health (ADH) sought to evaluate the uptake of COVID-19 vaccines within this priority group. In partnership with the Arkansas Department of Education (ADE), ADH received a list of 66,076 certified staff, classified staff, and teachers within the public K-12 school system. This list was matched to the state immunization registry via deterministic methods across three identifiers: first name, last name and date of birth. Uptake was assessed and the population was characterized using descriptive analyses. After 13 weeks of availability, 34,783 (51.2 %) of public K-12 teachers and staff had received at least one dose and 29,870 (44.0 %) had completed the series. School districts with the least robust uptake of COVID-19 vaccines tended to be in more rural areas, with some districts having less than 10 % of teachers and staff with at least one dose. The proportion of public K-12 teachers and staff with at least one dose of any COVID-19 vaccine grew quickly between January 18th and February 14th (4 % to 43 %) but has plateaued in the most recent seven weeks (45 % to 51 %). Although not directly measured, it is possible that vaccine hesitancy could be a factor in the attenuated uptake of COVID-19 vaccines within certain factions of the Arkansas public K-12 teacher and staff population. Overcoming vaccine hesitancy during the COVID-19 vaccine rollout will be critical in bringing an end to the pandemic.
Keywords: COVID-19; K-12; Vaccine; Vaccine hesitancy.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
[Technical guidelines for seasonal influenza vaccination in China (2021-2022)].Zhonghua Liu Xing Bing Xue Za Zhi. 2021 Oct 10;42(10):1722-1749. doi: 10.3760/cma.j.cn112338-20210913-00732. Zhonghua Liu Xing Bing Xue Za Zhi. 2021. PMID: 34814607 Chinese.
-
[Technical guidelines for seasonal influenza vaccination in China (2022-2023)].Zhonghua Liu Xing Bing Xue Za Zhi. 2022 Oct 10;43(10):1515-1544. doi: 10.3760/cma.j.cn112338-20220825-00734. Zhonghua Liu Xing Bing Xue Za Zhi. 2022. PMID: 36456484 Chinese.
-
[Technical guidelines for seasonal influenza vaccination in China (2022-2023)].Zhonghua Yu Fang Yi Xue Za Zhi. 2022 Oct 6;56(10):1356-1386. doi: 10.3760/cma.j.cn112150-20220825-00840. Zhonghua Yu Fang Yi Xue Za Zhi. 2022. PMID: 36274602 Chinese.
-
Barriers to COVID-19 Vaccines and Strategies to Improve Acceptability and Uptake.J Pharm Pract. 2023 Aug;36(4):900-904. doi: 10.1177/08971900221081621. Epub 2022 Apr 23. J Pharm Pract. 2023. PMID: 35465688 Free PMC article. Review.
-
COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines.Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review.
Cited by
-
CATCH-UP vaccines: protocol for a randomized controlled trial using the multiphase optimization strategy (MOST) framework to evaluate education interventions to increase COVID-19 vaccine uptake in Oklahoma.BMC Public Health. 2023 Jun 14;23(1):1146. doi: 10.1186/s12889-023-16077-w. BMC Public Health. 2023. PMID: 37316843 Free PMC article.
-
COVID-19 Vaccine Hesitancy among New Jersey Teachers and Impacts of Vaccination Information Dissemination.Vaccines (Basel). 2023 Feb 17;11(2):466. doi: 10.3390/vaccines11020466. Vaccines (Basel). 2023. PMID: 36851344 Free PMC article.
References
-
- Wallace M WK, Gargano JW, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine in Adolescents Aged 12–15 Years — United States, May 2021. MMWR Morb Mortal Wkly Rep 2021;ePub: 14 May 2021. doi:http://dx.doi.org/10.15585/mmwr.mm7020e1 external icon. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

