Directing the Self-Assembly of Aromatic Foldamer Helices using Acridine Appendages and Metal Coordination
- PMID: 35965255
- PMCID: PMC9826129
- DOI: 10.1002/chem.202201345
Directing the Self-Assembly of Aromatic Foldamer Helices using Acridine Appendages and Metal Coordination
Abstract
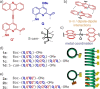
Folded molecules provide complex interaction interfaces amenable to sophisticated self-assembly motifs. Because of their high conformational stability, aromatic foldamers constitute suitable candidates for the rational elaboration of self-assembled architectures. Several multiturn helical aromatic oligoamides have been synthesized that possess arrays of acridine appendages pointing in one or two directions. The acridine units were shown to direct self-assembly in the solid state via aromatic stacking leading to recurrent helix-helix association patterns under the form of discrete dimers or extended arrays. In the presence of Pd(II), metal coordination of the acridine units overwhelms other forces and generates new metal-mediated multihelical self-assemblies, including macrocycles. These observations demonstrate simple access to different types of foldamer-containing architectures, ranging from discrete objects to 1D and, by extension, 2D and 3D arrays.
Keywords: aromatic stacking; foldamer; helical conformation; metal coordination; self-assembly.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

