Osteoclast-derived extracellular vesicles are implicated in sensory neurons sprouting through the activation of epidermal growth factor signaling
- PMID: 35965312
- PMCID: PMC9375906
- DOI: 10.1186/s13578-022-00864-w
Osteoclast-derived extracellular vesicles are implicated in sensory neurons sprouting through the activation of epidermal growth factor signaling
Abstract
Background: Different pathologies, affecting the skeletal system, were reported to display altered bone and/or cartilage innervation profiles leading to the deregulation of the tissue homeostasis. The patterning of peripheral innervation is achieved through the tissue-specific expression of attractive or repulsive axonal guidance cues in specific space and time frames. During the last decade, emerging findings attributed to the extracellular vesicles (EV) trading a central role in peripheral tissue innervation. However, to date, the contribution of EV in controlling bone innervation is totally unknown.
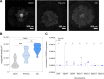
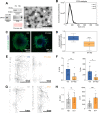
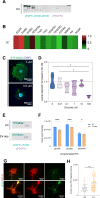
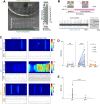
Results: Here we show that sensory neurons outgrowth induced by the bone resorbing cells-osteoclasts-is promoted by osteoclast-derived EV. The EV induced axonal growth is achieved by targeting epidermal growth factor receptor (EGFR)/ErbB2 signaling/protein kinase C phosphorylation in sensory neurons. In addition, our data also indicate that osteoclasts promote sensory neurons electrophysiological activity reflecting a possible pathway in nerve sensitization in the bone microenvironment, however this effect is EV independent.
Conclusions: Overall, these results identify a new mechanism of sensory bone innervation regulation and shed the light on the role of osteoclast-derived EV in shaping/guiding bone sensory innervation. These findings provide opportunities for exploitation of osteoclast-derived EV based strategies to prevent and/or mitigate pathological uncontrolled bone innervation.
Keywords: Bone pain; Epidermal growth factor receptor (EGFR/ErbB2) signaling; Extracellular vesicles; Neuronal electrophysiology; Osteoclast secretome; Sensory neurons sprouting.
© 2022. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures

Similar articles
-
Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain.J Clin Invest. 2019 Mar 1;129(3):1076-1093. doi: 10.1172/JCI121561. Epub 2019 Feb 4. J Clin Invest. 2019. PMID: 30530994 Free PMC article.
-
Osteoblasts are inherently programmed to repel sensory innervation.Bone Res. 2020 May 13;8:20. doi: 10.1038/s41413-020-0096-1. eCollection 2020. Bone Res. 2020. PMID: 32435517 Free PMC article.
-
Osteoblast-Derived Extracellular Vesicles Are Biological Tools for the Delivery of Active Molecules to Bone.J Bone Miner Res. 2018 Mar;33(3):517-533. doi: 10.1002/jbmr.3332. Epub 2017 Dec 11. J Bone Miner Res. 2018. PMID: 29091316
-
Regulation of osteoclast function via Rho-Pkn3-c-Src pathways.J Oral Biosci. 2019 Sep;61(3):135-140. doi: 10.1016/j.job.2019.07.002. Epub 2019 Aug 7. J Oral Biosci. 2019. PMID: 31400545 Review.
-
Osteoclast-Derived Extracellular Vesicles: Novel Regulators of Osteoclastogenesis and Osteoclast-Osteoblasts Communication in Bone Remodeling.Front Physiol. 2018 May 30;9:628. doi: 10.3389/fphys.2018.00628. eCollection 2018. Front Physiol. 2018. PMID: 29910740 Free PMC article. Review.
Cited by
-
M2 macrophage-derived cathepsin S promotes peripheral nerve regeneration via fibroblast-Schwann cell-signaling relay.J Neuroinflammation. 2023 Nov 9;20(1):258. doi: 10.1186/s12974-023-02943-2. J Neuroinflammation. 2023. PMID: 37946211 Free PMC article.
-
Mesenchymal Stem Cell-Derived Extracellular Vesicles in Bone-Related Diseases: Intercellular Communication Messengers and Therapeutic Engineering Protagonists.Int J Nanomedicine. 2024 Apr 6;19:3233-3257. doi: 10.2147/IJN.S441467. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38601346 Free PMC article. Review.
-
Epidermal Growth Factor Receptor Kinase Inhibitor Ameliorates β-Amyloid Oligomer-Induced Alzheimer Disease in Swiss Albino Mice.Molecules. 2022 Aug 14;27(16):5182. doi: 10.3390/molecules27165182. Molecules. 2022. PMID: 36014421 Free PMC article.
-
Decoding Pain: Next-Generation In Vitro Systems for Mechanistic Insights and Drug Discovery.FASEB J. 2025 Aug 31;39(16):e70914. doi: 10.1096/fj.202501025RR. FASEB J. 2025. PMID: 40814286 Free PMC article. Review.
-
Inhibition of inflammatory osteoclasts accelerates callus remodeling in osteoporotic fractures by enhancing CGRP+TrkA+ signaling.Cell Death Differ. 2024 Dec;31(12):1695-1706. doi: 10.1038/s41418-024-01368-5. Epub 2024 Sep 2. Cell Death Differ. 2024. PMID: 39223264 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

