Homozygous mutation of the LRRK2 ROC domain as a novel genetic model of parkinsonism
- PMID: 35965315
- PMCID: PMC9375908
- DOI: 10.1186/s12929-022-00844-9
Homozygous mutation of the LRRK2 ROC domain as a novel genetic model of parkinsonism
Abstract
Background: Parkinson's disease (PD) is one of the most important neurodegenerative disorders in elderly people. Mutations in the leucine-rich repeat kinase 2 (LRRK2) gene are found in a large proportion of the patients with sporadic and familial PD. Mutations can occur at different locations in the LRRK2. Patients with LRRK2 ROC-COR mutations face an increased risk of typical motor symptoms of PD, along with cognitive decline. An animal model with a monogenic LRRK2 gene mutation is a suitable model for exploring the pathophysiology of PD and identifying potential drug therapies. However, the effect of homozygous (HOM) LRRK2 in PD pathophysiology is unclear.
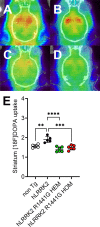
Methods: We established human LRRK2 (hLRRK2) R1441G HOM transgenic (Tg) mice to explore the phenotype and pathological features that are associated with hLRRK2 R1441G Tg mouse models and discuss the potential clinical relevance. The open field test (OFT) was performed to examine motor and nonmotor behaviors. A CatWalk analysis system was used to study gait function. [18F]FDOPA PET was used to investigate functional changes in the nigrostriatal pathway in vivo. Transmission electron microscopy was used to examine the morphological changes in mitochondria and lysosomes in the substantia nigra.
Results: The R1441G HOM Tg mice demonstrated gait disturbance and exhibited less anxiety-related behavior and exploratory behavior than mice with hLRRK2 at 12 months old. Additionally, [18F]FDOPA PET showed a reduction in FDOPA uptake in the striatum of the HOM Tg mice. Notably, there was significant lysosome and autophagosome accumulation in the cytoplasm of dopaminergic neurons in R1441G hemizygous (HEM) and HOM mice. Moreover, it was observed using transmission electron microscopy (TEM) that the mitochondria of R1441G Tg mice were smaller than those of hLRRK2 mice.
Conclusion: This animal provides a novel HOM hLRRK2 R1441G Tg mouse model that reproduces some phenotype of Parkinsonism in terms of both motor and behavioral dysfunction. There is an increased level of mitochondrial fission and no change in the fusion process in the group of HOM hLRRK2 R1441G Tg mouse. This mutant animal model of PD might be used to study the mechanisms of mitochondrial dysfunction and explore potential new drug targets.
Keywords: Anxiety; Fission; GTPase activity; Gait; Homozygous; LRRK2; PET; Parkinsonism; R1441G.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Gene and MicroRNA transcriptome analysis of Parkinson's related LRRK2 mouse models.PLoS One. 2014 Jan 10;9(1):e85510. doi: 10.1371/journal.pone.0085510. eCollection 2014. PLoS One. 2014. PMID: 24427314 Free PMC article.
-
Diapocynin prevents early Parkinson's disease symptoms in the leucine-rich repeat kinase 2 (LRRK2R¹⁴⁴¹G) transgenic mouse.Neurosci Lett. 2013 Aug 9;549:57-62. doi: 10.1016/j.neulet.2013.05.034. Epub 2013 May 28. Neurosci Lett. 2013. PMID: 23721786 Free PMC article.
-
A novel mitochondrially-targeted apocynin derivative prevents hyposmia and loss of motor function in the leucine-rich repeat kinase 2 (LRRK2(R1441G)) transgenic mouse model of Parkinson's disease.Neurosci Lett. 2014 Nov 7;583:159-64. doi: 10.1016/j.neulet.2014.09.042. Epub 2014 Sep 26. Neurosci Lett. 2014. PMID: 25263790 Free PMC article.
-
LRRK2 mutant knock-in mouse models: therapeutic relevance in Parkinson's disease.Transl Neurodegener. 2022 Feb 14;11(1):10. doi: 10.1186/s40035-022-00285-2. Transl Neurodegener. 2022. PMID: 35152914 Free PMC article. Review.
-
[Clinical molecular genetics for PARK8 (LRRK2)].Brain Nerve. 2007 Aug;59(8):839-50. Brain Nerve. 2007. PMID: 17713120 Review. Japanese.
Cited by
-
Axonal energy metabolism, and the effects in aging and neurodegenerative diseases.Mol Neurodegener. 2023 Jul 20;18(1):49. doi: 10.1186/s13024-023-00634-3. Mol Neurodegener. 2023. PMID: 37475056 Free PMC article. Review.
-
LRRK2-mediated mitochondrial dysfunction in Parkinson's disease.Biochem J. 2025 May 28;482(11):721-39. doi: 10.1042/BCJ20253062. Biochem J. 2025. PMID: 40440058 Free PMC article. Review.
-
The homozygous LRRK2.p.N1437D point mutation mouse is a novel model of parkinsonism.NPJ Parkinsons Dis. 2025 Mar 28;11(1):61. doi: 10.1038/s41531-025-00905-4. NPJ Parkinsons Dis. 2025. PMID: 40155632 Free PMC article.
-
Deficiency of miR-29a/b1 leads to premature aging and dopaminergic neuroprotection in mice.Front Mol Neurosci. 2022 Oct 6;15:978191. doi: 10.3389/fnmol.2022.978191. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36277485 Free PMC article.
References
-
- Alcalay RN, Mirelman A, Saunders-Pullman R, Tang MX, Mejia Santana H, Raymond D, Roos E, Orbe-Reilly M, Gurevich T, Bar Shira A, Gana Weisz M, Yasinovsky K, Zalis M, Thaler A, Deik A, Barrett MJ, Cabassa J, Groves M, Hunt AL, Lubarr N, San Luciano M, Miravite J, Palmese C, Sachdev R, Sarva H, Severt L, Shanker V, Swan MC, Soto-Valencia J, Johannes B, Ortega R, Fahn S, Cote L, Waters C, Mazzoni P, Ford B, Louis E, Levy O, Rosado L, Ruiz D, Dorovski T, Pauciulo M, Nichols W, Orr-Urtreger A, Ozelius L, Clark L, Giladi N, Bressman S, Marder KS. Parkinson disease phenotype in Ashkenazi Jews with and without LRRK2 G2019S mutations. Mov Disord. 2013;28(14):1966–1971. doi: 10.1002/mds.25647. - DOI - PMC - PubMed
-
- Alegre-Abarrategui J, Christian H, Lufino MM, Mutihac R, Venda LL, Ansorge O, Wade-Martins R. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum Mol Genet. 2009;18(21):4022–4034. doi: 10.1093/hmg/ddp346. - DOI - PMC - PubMed
-
- Bajpai B. High capacity vectors. In: Ravi I, Baunthiyal M, Saxena J, editors. Advances in Biotechnology. Berlin: Springer; 2014. pp. 1–10.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

